According to Bohr.s theory, En = total energy, Kn = kinetic energy , Vn = potential energy, rn = radius of nth orbit. Match the following.
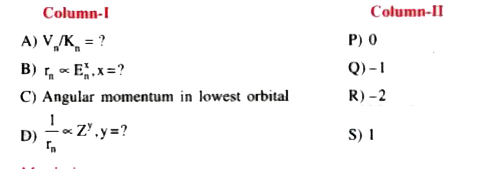 Solution in Telugu
Solution in Telugu
The correct Answer is:A-R; B-Q; C-P; D-S
Step by step video, text & image solution for According to Bohr.s theory, E_(n) = total energy, K_(n) = kinetic energy , V_(n) = potential energy, r_(n) = radius of n^(th) orbit. Match the following. by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
बोहर सिद्धांत के अनुसार Ea = पूर्ण ऊर्जा, Ka = गतिज ऊर्जा, Vn = स्थितिज ऊर्जा, rn=nth कक्षा की त्रिज्या निम्न को मिलाइये।

View SolutionIf in Bohr.s model, for uni electronic atom following symbols are used :
rn,Z→ Radius of nth orbit with atomic number z
Un,Z→ Potential energy of e−
Kn,Z→ Kinetic energy of e−
Vn,Z→ Velocity of e−
Tn,Z→ Time period of revolution

View SolutionAccording to Bohr.s theory, En = total energy, Kn = kinetic energy, Vn = potential energy, rn = radius of nth orbit. Match the following.

View SolutionIf in Bohr.s model, for uni electronic atom following symbols are used : r_(n,z) to Radius of n^(th) orbit with atomic number z. U_(n.z) to Potential energy of e^- K_(n.Z) to Kinetic energy of e^- T_(n.Z) to Time period of revolution
View SolutionIf E_(p) and E_(x) represent potential energy and kinetic energy.respectively, of an orbital electron, then, according to Bohr.s theory :
View SolutionIf EpandEk represent potential energy and kinetic energy respectively of an orbital electron, then, according to Bohr.s theory :
View SolutionNth level of Li2+ has the same energy as the ground state energy of the hydrogen atom. If rN and r1 be the radius of the Nth Bohr orbit of Li2+ and first orbit radius of H atom respectively, then the ratio rNr1 is
View Solution

