Ground state electronic configuration of p atom can be represented as Solution in Telugu
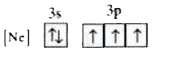


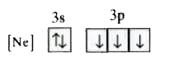
The correct Answer is:A, B, C, D
Step by step video, text & image solution for Ground state electronic configuration of p atom can be represented as by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- ATOMIC STRUCTUREBook:AAKASH SERIESChapter:ATOMIC STRUCTUREExercise:Level - II (Type-I) (Practice sheet-5 (Section - B : Linked comprehension Type questions))
Explore 5 Videos
- ATOMIC STRUCTUREBook:AAKASH SERIESChapter:ATOMIC STRUCTUREExercise:Level - II (Type-I) (Practice sheet-5 (Section -C : Matching/Straight objective type questions))
Explore 2 Videos
- ATOMIC STRUCTUREBook:AAKASH SERIESChapter:ATOMIC STRUCTUREExercise:Level - II (Type-I) (Lacture sheet - 5 (Section -C : Matching/Straight objective type questions))
Explore 2 Videos
- AROMATIC HYDROCARBONSBook:AAKASH SERIESChapter:AROMATIC HYDROCARBONSExercise:OBJECTIVE EXERCIES - 3 (RECENT AIPMT/NEET QUESTIONS)
Explore 10 Videos
- CHEMICAL BONDINGBook:AAKASH SERIESChapter:CHEMICAL BONDINGExercise:OBJECTIVE EXERCISE -3 (RECENT AIPMT/NEET QUESTIONS )
Explore 39 Videos
Similar Questions
The equation of Schroedinger for the hydrogen atom in the time-independet, non-relativistic form is a partial differential equation involving the position coordinates (x, y and z). The potential energy term for the proton-electron system is spherically symmetric of the form −1/4π∈0×(e2/r). THus it is advantages to change over from the cartesian coordinates (x,y and z) to the spherical polar coordinates, (r,θ and ϕ). In this form the equation become separable in the radial part involving r and the angular part involving θ and ϕ. The probability of locating the electron within a volume element dτ=4πr2dr is then given |Ψ|2(4πr2dr), where Ψ is a function of r, θ and ϕ. With proper conditions imposed on Ψ, the treatment yields certain functions, Ψ, known as atomic orbitals which are solutions of the equations. Each function Ψ correspods to quantum number n, l and m, the principal, the azimuthal and the magnetic quantum number respectively, n has values 1, 2, 3,...., l has values 0, 1, 2, ....(n-1) for each value of n and m (n-1) for each value of n and m (ml) has values =1, +(l+1),...1,0,-1,-2...-l i.e., (2l+1) values for each value of l. In addition a further quantum number called pin had to be introduced with values ±1/2. Any set of four values for n, l , m and s characterizes a spin orbital. Pauli.s exclusion principle states that a given spin orbital can accomodate not more than electron. Further the values l = 0, l=1, l=2, l=3 are designated s,p,d and f orbitals respectively.
How many spin orbitals are there corresponding to n = 3?
View SolutionMatch the following

View SolutionMatch the following

View SolutionUsing anhydrous AICI3 as catalyst which one of the following reactions produces ethyl benzene
View SolutionIn which of the following pairs, the two species are isostructural?
View SolutionWhich of the following can be acceptable electronic configuration of carbon atom in the ground state?
View SolutionWhich of the following options does not represent ground state electronic configuration of an atom?
View SolutionGround state electronic configuration of nitrogen atom can be represented by:
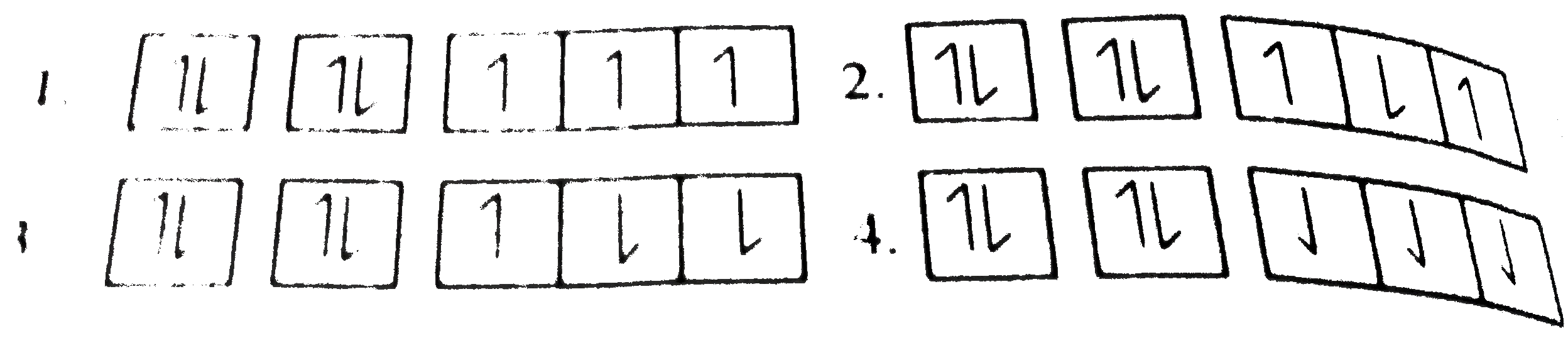
View SolutionThe ground state electronic configuration of nitrogen atom can be represented as:
View SolutionGround state electronic configuration of nitrogen atom can be represented by
View SolutionGround state electronic configuration of nitrogen atom can be represented by:
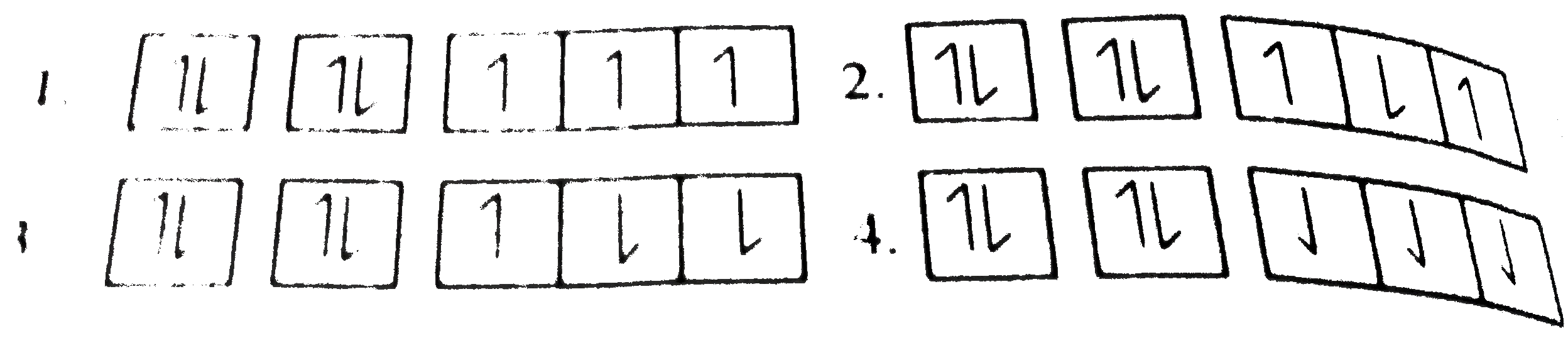
View SolutionWhich of the following options does not represent ground state electronic configuration of an atom?
View SolutionWhich of the following do not represent ground state electronic configuration of an atom ?
View SolutionWhich of the following options does not represent ground state electronic configuration of an atom?
View SolutionWhich of the following options does not represent ground state electronic configuration of an atom ?
View Solution
