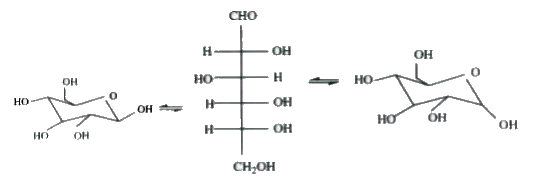
D(+) Glucose has melting point 140∘C and specific rotation [a]25D is 112∘C. Another D(+) Glucose has melting point 150∘C and specific rotation [a]25D is +18.7∘C. The two form have significantly different optical rotation but when an aqueous solution of either form is allowed to stand, it rotation changes. The specific rotation of one form decreases and rotation of other increases until both solution show the same value +52.7∘. The change in rotation towards an equilibrium value is called mutarotation
^(25) is 112^(@)C. Another D(+) Glucose has melting point 150^(@)C and specific rotation [a](D)^(25) is +18.7^(@)C. The two form have significantly different optical rotation but when an aqueous solution of either form is allowed to stand, it rotation changes. The specific rotation of one form decreases and rotation of other increases until both solution show the same value +52.7^(@). The change in rotation towards an equilibrium value is called mutarotation [alpha](D)^(25) = +18.7^(@)C](https://d10lpgp6xz60nq.cloudfront.net/physics_images/AKS_TRG_AO_CHE_XII_V02_A_C01_E05_017_Q01.png)
[α]25D=+18.7∘C [α]25D=+112∘C
Mutarotation is characteristic feature ofSolution in Telugu
Epimer
Enantiomer
Anomer
Ring chain isomer
The correct Answer is:C
Mutarotation
Similar Questions
Glucose shows mutarotation when it dissolves in water. The specific rotation of α-D glucose and β-D- glucose is +112.2∘ and +18.7∘ respectively. Calculate the percentage of two anomers present at equilibrium mixture with a specific rotation of +52.6∘.
View SolutionForeword: Freshly made alpha - Specific rotation of glucose solution + 112^(@) From 52.7^(@) While lowering beta Specific rotation of freshly made solution of glucose + 19^(@) From 52.7^(@) Rises to Reason: of an optically active compound. The change in specific rotation over time up to the equilibrium value is called mutarotation.
View SolutionD(+) Glucose has melting point 140∘C and specific rotation [a]25D is 112∘C. Another D(+) Glucose has melting point 150∘C and specific rotation [a]25D is +18.7∘C. The two form have significantly different optical rotation but when an aqueous solution of either form is allowed to stand, it rotation changes. The specific rotation of one form decreases and rotation of other increases until both solution show the same value +52.7∘. The change in rotation towards an equilibrium value is called mutarotation
[α]25D=+18.7∘C [α]25D=+112∘C
What percentage of β−D−(+) glucopyranose found at equilibrium in the aqueous solution?
View SolutionD(+) Glucose has melting point 140^(@)C and specific rotation [a]_(D)^(25) is 112^(@)C . Another D(+) Glucose has melting point 150^(@)C and specific rotation [a]_(D)^(25) is +18.7^(@)C . The two form have significantly different optical rotation but when an aqueous solution of either form is allowed to stand, it rotation changes. The specific rotation of one form decreases and rotation of other increases until both solution show the same value +52.7^(@) . The change in rotation towards an equilibrium value is called mutarotation [alpha]_(D)^(25) = +18.7^(@)C " " [alpha]_(D)^(25) = +112^(@)C For mannose the mutarotation can be shown in brief as follow
View SolutionA sample of optically active alcohol has a specific rotation, [α]25D, equal to +1.151∘. Specific rotation for pure form [α]25D is +5.756∘.
What is the percentage enantiomeric excess of the sample?
View SolutionWhen either form of D-Glucose is dissolved In water, the solution gradually changes its optical rotation and finally attains a constant optical rotation of +52^(@) . Which form of D-GIucose is more stable?
View SolutionWhen either form of D-Glucose is dissolved In water, the solution gradually changes its optical rotation and finally attains a constant optical rotation of +52∘.
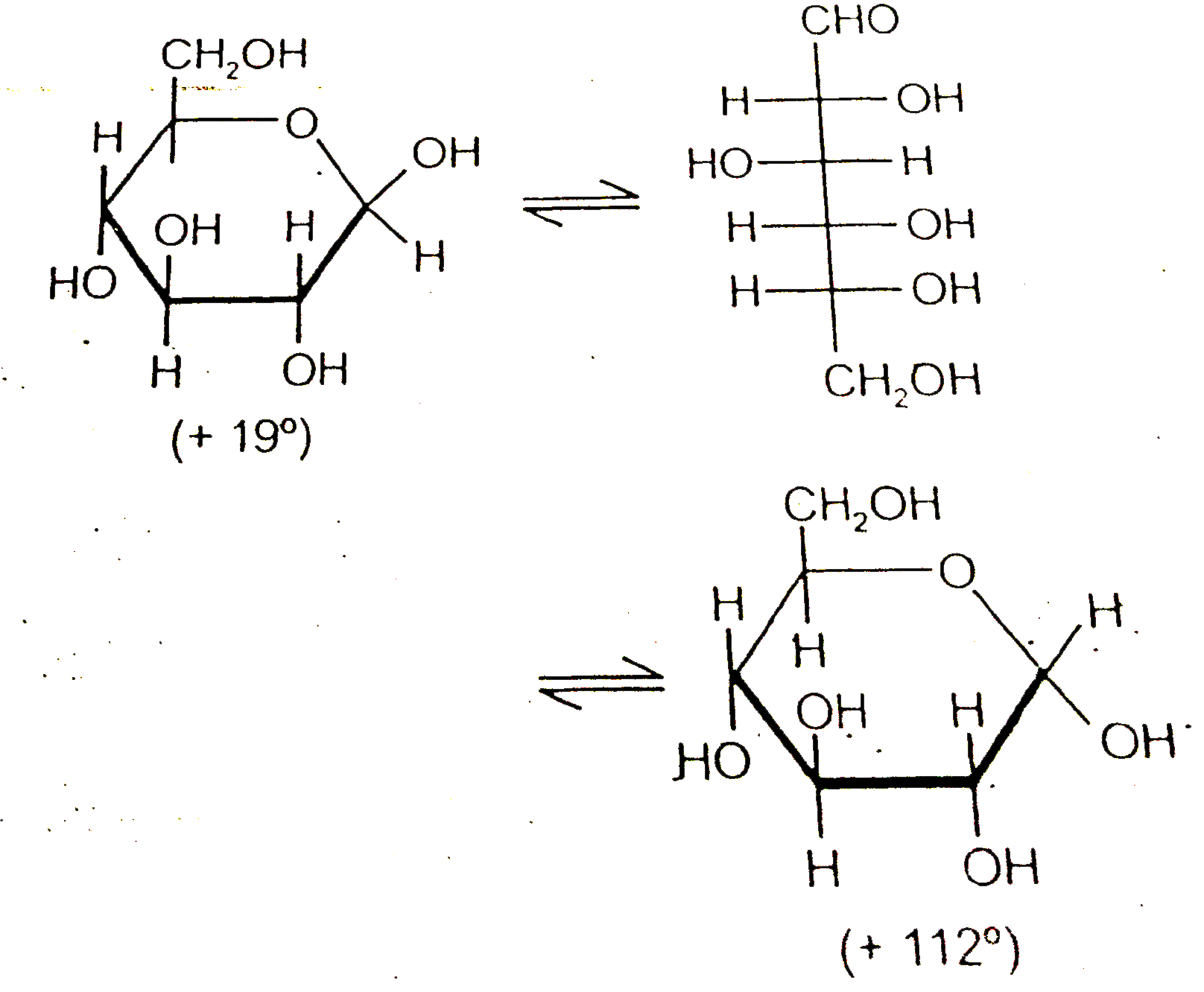
What is the percentage of open chain D-Glucose in solution?
View Solution

