Find the average molecular mass of starch given that an aqueous solution of10g/c of starch has an osmatic pressure 5×10−3 atm at 25∘C what is the approximate number of glucose units in this sample of starchSolution in Telugu
312
302
322
332
The correct Answer is:B
Concentration of glucose =C=104gmM,π=CRT,5×10−3=104M,x0.0821×298∴M=4.893×107
Number of glucose units =4.893×107162=302 (Note : MW of glucose is taken as 162 since in the formation of starch H2O is lost 180−18=162)
Topper's Solved these Questions
- CARBOHYDRATESBook:AAKASH SERIESChapter:CARBOHYDRATESExercise:Practice Sheet-4
Explore 30 Videos
- BIOMOLECULESBook:AAKASH SERIESChapter:BIOMOLECULESExercise:OBJECTIVE EXERCISE-4 (ASSERTION (A) & REASON (R) TYPE QUESTIONS :)
Explore 84 Videos
- CARBOXYLIC ACIDS Book:AAKASH SERIESChapter:CARBOXYLIC ACIDS Exercise:PRACTICE SHEET-4 (Integer Type Questions)
Explore 9 Videos
Similar Questions
Sugars are characterized by the preparation of Osazone derivatives. Which sugars have identical osazones
View Solution(A) Fructose has ketone group and does not reduce Tollen's reagent.
(R) Fructose in Haworth structure has SiX membered ring.
View SolutionHow many of the following compounds will give Hoffman Hypo bromide reaction.
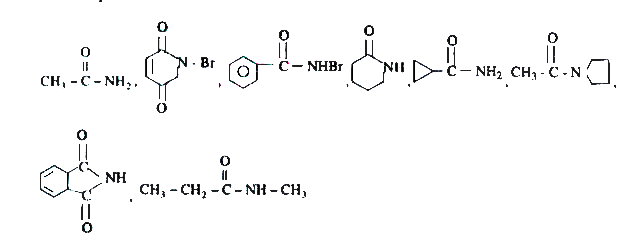
View Solution(a). What is the average molecular mass of starch?
(b). What is the approximate average number of glucose units in the sample of starch?
(Given:20gmL−1 of starch has an osmotic pressure =10−2atmat25∘C.)
View SolutionCalculating osmotic pressure: The fomula for low molecular mass starch is (C_(6)H_(10)O_(5))_(n) , where n averages 2.00xx10^(2) . When 0.798g of starch is dissolved in 100.0 mL of water solution, what is the osmotic pressure at 25^(@)C ? Strategy: Work out the molecular mass of (C_(6)H_(10)O_(5))_(200) , and use it to obtain the molarity of the starch solution. Substitute into the formula for the osmotic pressure, pi=CRT .
View SolutionHydrolysis of starch into glucose is a/an
View Solution(i) Starch is a polymer of (ii) basic unit of starch is
View SolutionAssertion : Starch is the storage polysaccharide in plants . Reason : Starch is a polymer of beta - glucose .
View SolutionCellulose, glucose, starch, ghee.
View Solutionखाद्य पदार्थ के नमूने में स्टार्च की उपस्तिथि की जांच के लिए इस्तेमाल किया जाने वाला विलयन है
View Solutionस्टार्च का एकलक (Monomer ) इकाई ग्लूकोस होता है।
View SolutionHow to get Starch to Glucose
View SolutionThe amylose portion of starch has a glycoside linkage involved in the addition of glucose units.
View Solutionসালোকসংশ্লেষে উৎপন্ন গ্লুকোজ, শ্বেতসার বা স্টার্চে রপান্তরিত হয় কেন?
View SolutionStarch is polymer of:
View Solution
