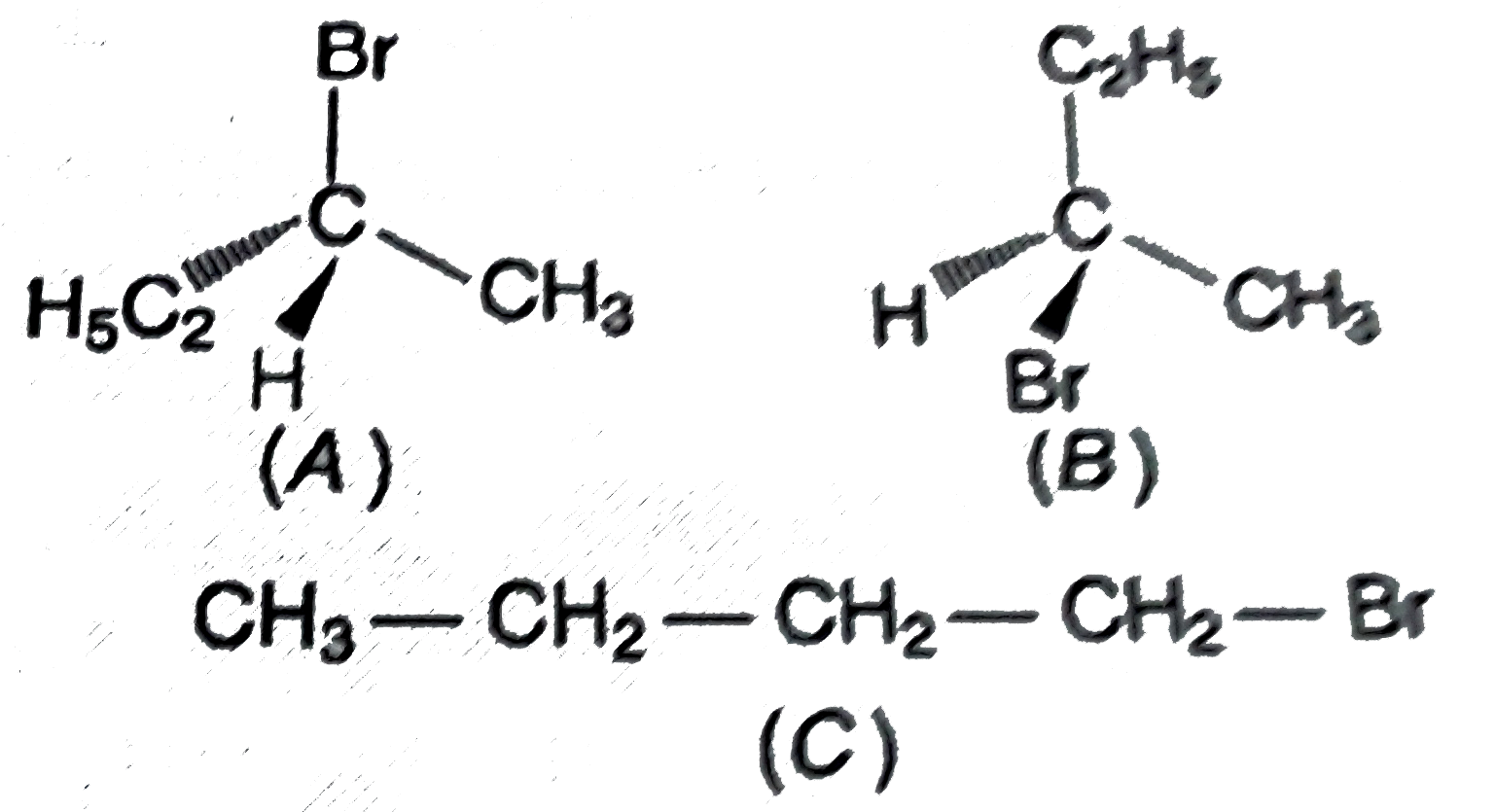
The addition of HBr of 1-butene gives a mixture of products A,B and C
(C) CH3−CH2−CH2−CH2−Br
The mixture consists of
A and B as major and C as minor products
B as major, A and C as major products
B as minor , A and C as major products
A and B as minor and C as major products
The correct Answer is:A
The alkene is unsymmetrical, hence will follow Markownikoff's rule to give major product.
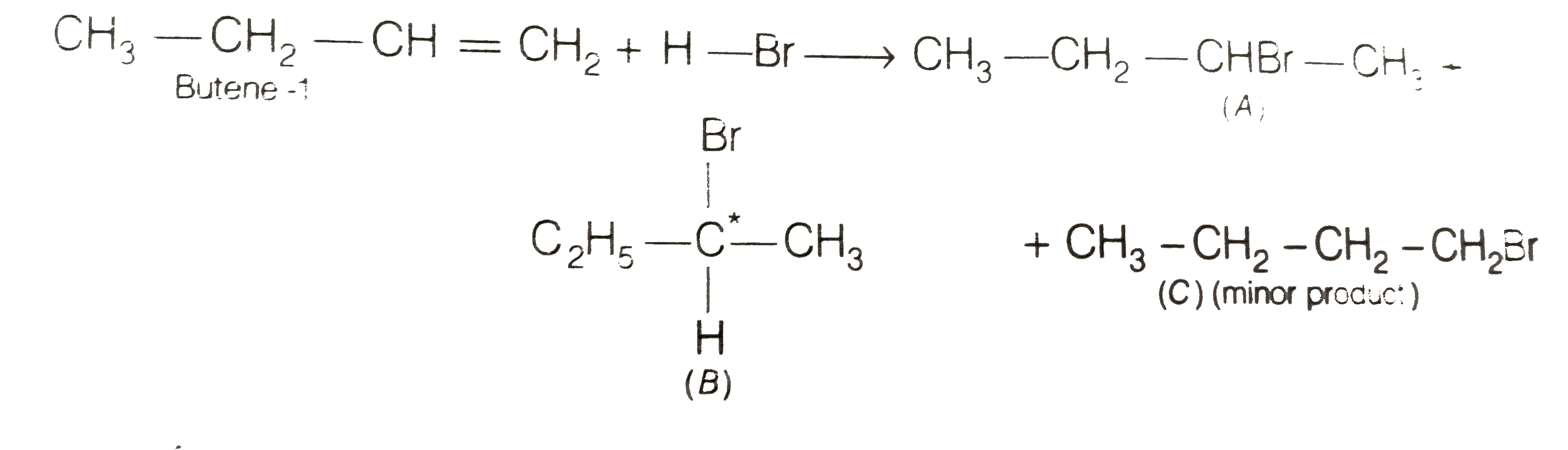
+CH3−CH2−CH2−CH2Br(C)(minor product)
Since, I contains , a chiral carbon , it exists in two enantiomers (A and B) wich are mirror images of other.
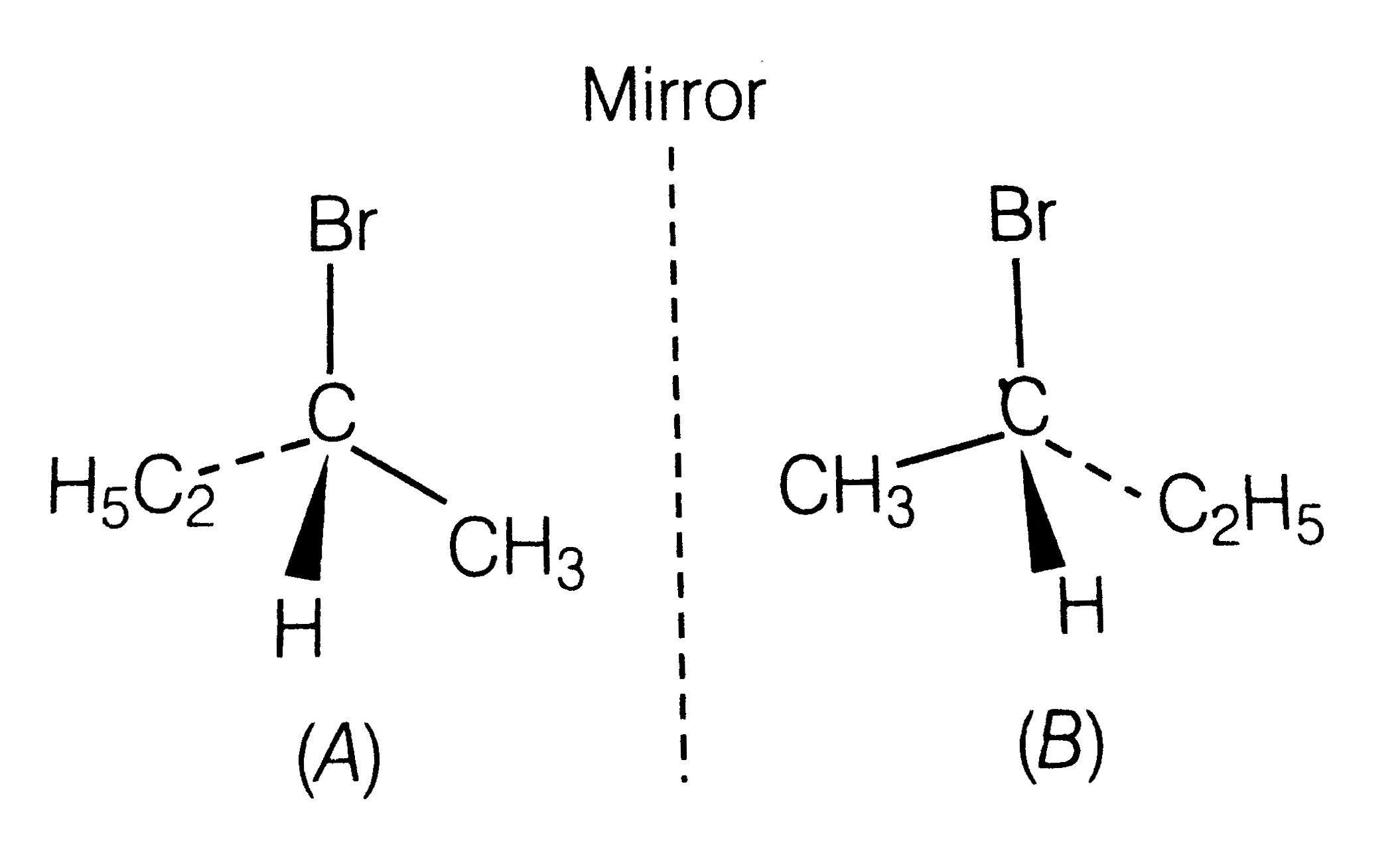
Similar Questions
Knowledge Check
Br−CH2−(CH2)2−CH2−Br+CH3NH2→ Product of the reaction is :
A B
B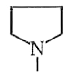 C
C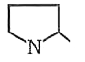 D
D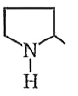
Give the IUPAC name of the compound
(CH3)3C−CH2−CH2−CH2−CH2−C(CH3)3
View SolutionIdentify the major product A. CH3−CH=CH−CH=CH2HBr−−−−→(40∘C)A
View SolutionWrite the strucutre of the product and mechanism of the following reaction: (CH3)3C−CH2OHHBr−−→
View SolutionCH3−O−CH2−PhHBr−−→ ? Product is
View SolutionWhich alkyl halide from the following pairs would you expect to react more rapidly by an SN2 mechanism? Explain your answer.
(i)CH3CH2CH2CH2BrorCH3CH2C∣BrHCH3,(ii)CH3CH2C∣BrHCH3orH3C−CH3∣C∣CH3−Br
(iii)CH3C∣CH3HCH2CH2BrorCH3CH2C∣CH3HCH2Br
View SolutionIn the given compounds
CH3CH2CH2CH2Br (a)
CH3CH2CHBr−CH3 (b)
(CH3)3C−Br(c)
The correct order of boiling point is
(a)>(b)>(c)
(b)>(a)>(c)
(c)>(a)>(b)
(a)>(c)>(b)
View Solution(a) Which of the following are position isomers ?
(i) CH2=CH (ii) CH3−C−CH3 (iii) CH3−CH=C−CH3 | || | CH2−CH3 CH2 CH2
(b) Write the IUPAC name of the functional isomer of CH3CH2CH2CH2CHO.
(c) Which type of structural isomerism is shown by the following pair of compounds ?
Br CH3 | |CH3−CH−CHBr2andBr2CH−CH−Br
View Solution

