Among the elements, B, Al, C and Si,
a) which element has the highest first ionisation enthalpy?
b) which element has the most metallic character? Justify your answer in each case,
Video Solution
Text Solution
The correct Answer is:A
The placing of elements are as
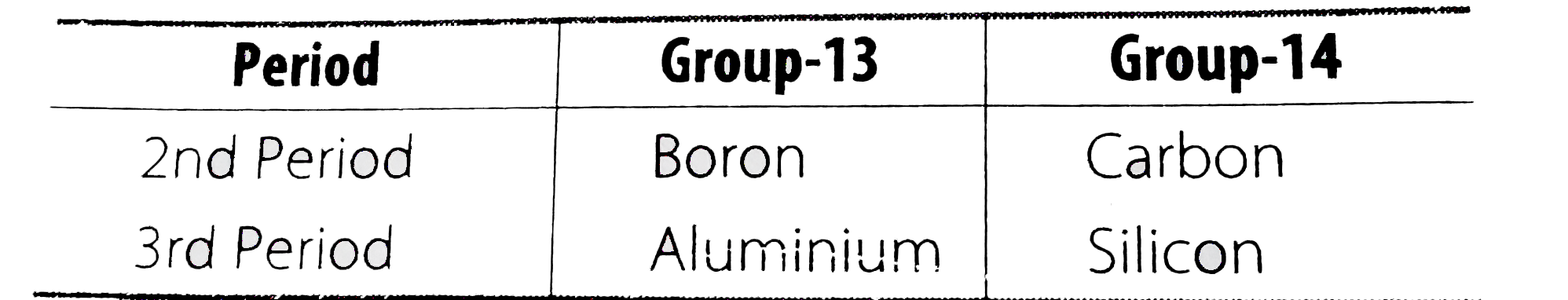
a) Ionisation enthalpy increased along a period (as we move from left to right in a period) with decrease in atomic size and decreases down the group with increase in atomic size. Hence, carbon has the highest first ionisation enthalpy.
b) Metallic character decreases across a period but increases on moving down the group. Hence, aluminium has the most mettalic character.
|
Similar Questions
Knowledge Check
Which group of elements shows lowest ionisation enthalpy?
AAlkali metalsBAlkaline earth metalsCHalogensDNoble gases
The first ionisation potential of which of the element is highest
View SolutionWhich elements has highest catenation property?
View SolutionThe element which has highest 2nd ionisation energy is
View SolutionAmong the elements of group 14, which element shows the highest tendency for catenation?
View SolutionWhich of the following element has the highest ionisation enregy ?
View SolutionAmong the following elements which one has the highest value of first ionisation potential ?
View SolutionAmong the elements of the third period (Na to Ar), pick out the element
with the highest first ionization enthalpy.
View Solution
