Haloalkanes contain halogen atom(s) attached to the sp3hybridised carbon atom of an alkyl group. Identify haloalkane from the following compounds.
2-bromopentane
Vinyl chloride (chloroethane)
2-chloroacetophenon
Trichloromethane
The correct Answer is:A, D
To identify the haloalkane from the given compounds, we need to understand that haloalkanes are compounds where a halogen atom is attached to an sp³ hybridized carbon atom of an alkyl group. Let's analyze each compound step by step.
1. Identify the first compound: 2-bromopentane
- The structure of 2-bromopentane consists of a five-carbon chain (pentane) with a bromine atom attached to the second carbon.
- The structure can be drawn as:
CH3−CH(Br)−CH2−CH2−CH3
- The second carbon is sp³ hybridized because it forms four single bonds (three with hydrogen and one with bromine).
- Conclusion: 2-bromopentane is a haloalkane.
2. Identify the second compound: Vinyl chloride
- The structure of vinyl chloride is:
CH2=CHCl
- The carbon atom to which chlorine is attached is involved in a double bond (sp² hybridized).
- Conclusion: Vinyl chloride is not a haloalkane.
3. Identify the third compound: 2-chloroacetophenone
- The structure of acetophenone is:
C6H5−C(=O)−CH3
- In 2-chloroacetophenone, chlorine is attached to the carbon adjacent to the carbonyl group:
C6H5−C(Cl)−C(=O)−CH3
- The carbon atom to which chlorine is attached is sp² hybridized (part of the benzene ring).
- Conclusion: 2-chloroacetophenone is not a haloalkane.
4. Identify the fourth compound: Trichloromethane (chloroform)
- The structure of trichloromethane is:
CHCl3
- The carbon atom in trichloromethane is bonded to three chlorine atoms and one hydrogen atom, forming four single bonds.
- This carbon is sp³ hybridized.
- Conclusion: Trichloromethane is a haloalkane.
Final Answer:
The haloalkanes from the given compounds are 2-bromopentane and trichloromethane.
- The structure of 2-bromopentane consists of a five-carbon chain (pentane) with a bromine atom attached to the second carbon.
- The structure can be drawn as:
CH3−CH(Br)−CH2−CH2−CH3
- The second carbon is sp³ hybridized because it forms four single bonds (three with hydrogen and one with bromine).
- Conclusion: 2-bromopentane is a haloalkane.
- The structure of vinyl chloride is:
CH2=CHCl
- The carbon atom to which chlorine is attached is involved in a double bond (sp² hybridized).
- Conclusion: Vinyl chloride is not a haloalkane.
- The structure of acetophenone is:
C6H5−C(=O)−CH3
- In 2-chloroacetophenone, chlorine is attached to the carbon adjacent to the carbonyl group:
C6H5−C(Cl)−C(=O)−CH3
- The carbon atom to which chlorine is attached is sp² hybridized (part of the benzene ring).
- Conclusion: 2-chloroacetophenone is not a haloalkane.
- The structure of trichloromethane is:
CHCl3
- The carbon atom in trichloromethane is bonded to three chlorine atoms and one hydrogen atom, forming four single bonds.
- This carbon is sp³ hybridized.
- Conclusion: Trichloromethane is a haloalkane.
The haloalkanes from the given compounds are 2-bromopentane and trichloromethane.
Topper's Solved these Questions
Similar Questions
Knowledge Check
Haloalkanes contain halogen atom(s) attached to the sp3hybridised carbon atom of an alkyl group. Identify haloalkene from the following compounds.
AOnly (ii)B(ii) and (iv) onlyC(i) and (iv) onlyD(i),(ii) and (iii) only
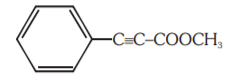
How many sp2 and sp-hybridised carbon atoms are present respectively in the following compound ?
View SolutionNumber of sp hybridised carbon atoms in But-2-yne is
View SolutionCan an organic compound have two OH groups attached to the same carbon atom ?
View SolutionAmong the following compounds, which has three sp-hybridised carbon atoms ?
View SolutionAll carbon atoms are sp2 hybridised in
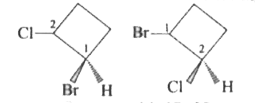
View SolutionThe configuration of 1 and 2 carbon atom in the following compounds is :
View SolutionThe central atom assume sp3 hybridisation in:
View Solution