Which of the following reaction are correct?
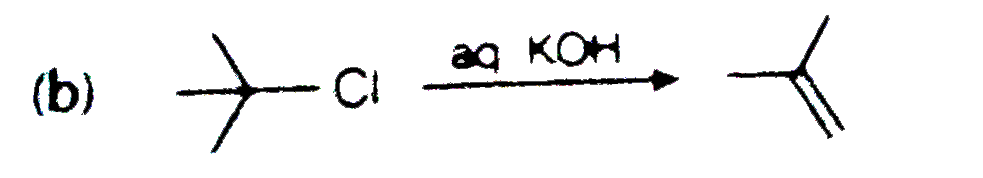
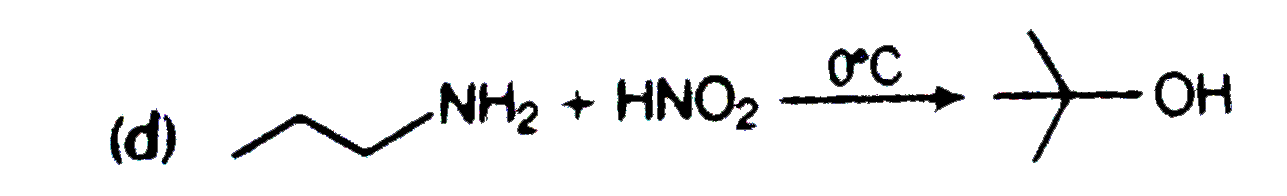
The correct Answer is:A, C
To determine which of the given reactions are correct, we will analyze each option step by step.
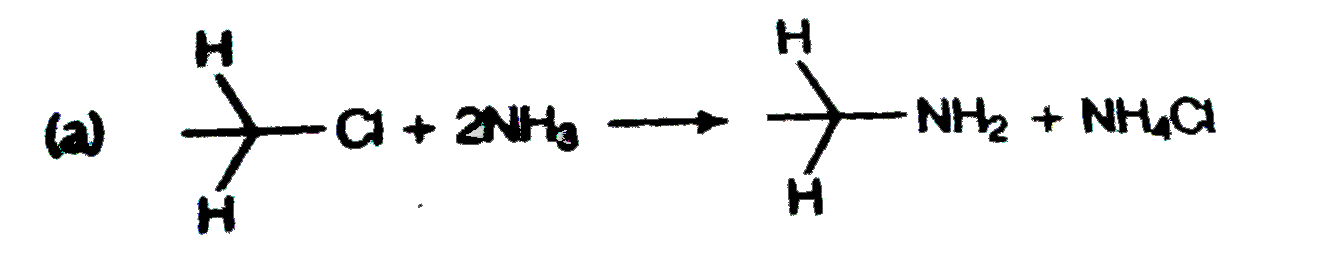
Step 1: Analyze Option A
- Reaction: Ethyl chloride (C2H5Cl) reacts with ammonia (NH3).
- Mechanism: This reaction proceeds via an SN2 mechanism, where the nucleophile (NH3) attacks the electrophilic carbon attached to the chlorine (Cl) atom.
- Product: The chlorine atom is replaced by an amino group (NH2), resulting in the formation of ethylamine (C2H5NH2) and ammonium chloride (NH4Cl) as a byproduct.
- Conclusion: This reaction is correct.
Step 2: Analyze Option B
- Reaction: A tertiary alcohol reacts with aqueous KOH.
- Mechanism: Aqueous KOH typically favors nucleophilic substitution (SN) rather than elimination. In this case, the chlorine (Cl) would be replaced by a hydroxyl group (OH) rather than undergoing elimination.
- Expected Product: The expected product should be a tertiary alcohol (with OH replacing Cl), not a double bond.
- Conclusion: This reaction is incorrect.
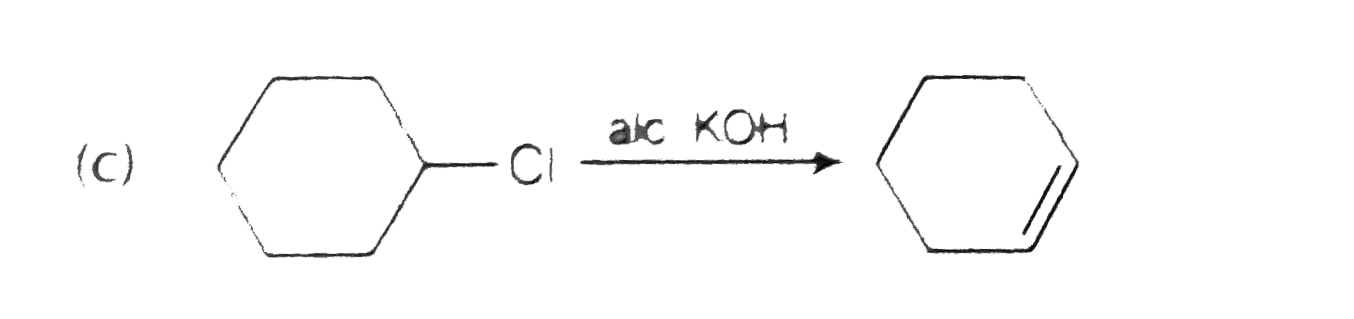
Step 3: Analyze Option C
- Reaction: Chlorocyclohexane reacts with alcoholic KOH.
- Mechanism: Alcoholic KOH promotes elimination reactions (dehydrohalogenation). In this case, both H and Cl are removed, leading to the formation of a double bond in cyclohexene.
- Product: The product is cyclohexene, which is formed by the elimination of HCl.
- Conclusion: This reaction is correct.
Step 4: Analyze Option D
- Reaction: A primary amine reacts with nitrous acid (HNO2).
- Mechanism: When a primary amine reacts with HNO2, it forms a diazonium salt (R-N2^+Cl^-) which is unstable in the aliphatic case. It will eventually convert to an alcohol (R-OH) upon hydrolysis.
- Expected Product: The product should be a primary alcohol, not a tertiary alcohol.
- Conclusion: This reaction is incorrect.
Final Conclusion
The correct reactions are:
- Option A: Correct
- Option B: Incorrect
- Option C: Correct
- Option D: Incorrect
Thus, the correct reactions are A and C.
---
- Reaction: Ethyl chloride (C2H5Cl) reacts with ammonia (NH3).
- Mechanism: This reaction proceeds via an SN2 mechanism, where the nucleophile (NH3) attacks the electrophilic carbon attached to the chlorine (Cl) atom.
- Product: The chlorine atom is replaced by an amino group (NH2), resulting in the formation of ethylamine (C2H5NH2) and ammonium chloride (NH4Cl) as a byproduct.
- Conclusion: This reaction is correct.
- Reaction: A tertiary alcohol reacts with aqueous KOH.
- Mechanism: Aqueous KOH typically favors nucleophilic substitution (SN) rather than elimination. In this case, the chlorine (Cl) would be replaced by a hydroxyl group (OH) rather than undergoing elimination.
- Expected Product: The expected product should be a tertiary alcohol (with OH replacing Cl), not a double bond.
- Conclusion: This reaction is incorrect.
- Reaction: Chlorocyclohexane reacts with alcoholic KOH.
- Mechanism: Alcoholic KOH promotes elimination reactions (dehydrohalogenation). In this case, both H and Cl are removed, leading to the formation of a double bond in cyclohexene.
- Product: The product is cyclohexene, which is formed by the elimination of HCl.
- Conclusion: This reaction is correct.
- Reaction: A primary amine reacts with nitrous acid (HNO2).
- Mechanism: When a primary amine reacts with HNO2, it forms a diazonium salt (R-N2^+Cl^-) which is unstable in the aliphatic case. It will eventually convert to an alcohol (R-OH) upon hydrolysis.
- Expected Product: The product should be a primary alcohol, not a tertiary alcohol.
- Conclusion: This reaction is incorrect.
The correct reactions are:
- Option A: Correct
- Option B: Incorrect
- Option C: Correct
- Option D: Incorrect
Topper's Solved these Questions
- AMINESBOOK - NCERT EXEMPLAR ENGLISHCHAPTER - AMINESEXERCISE - SATQ
37 Videos
- AMINESBOOK - NCERT EXEMPLAR ENGLISHCHAPTER - AMINESEXERCISE - LONG ANSWER
3 Videos
- ALDEHYDE, KETONES AND CARBOXYLIC ACIDSBOOK - NCERT EXEMPLAR ENGLISHCHAPTER - ALDEHYDE, KETONES AND CARBOXYLIC ACIDSEXERCISE - Long Answer Type Questions
4 Videos
- BIOMOLECULES BOOK - NCERT EXEMPLAR ENGLISHCHAPTER - BIOMOLECULES EXERCISE - Long Answer Type Questions
5 Videos
Similar Questions
What is the role of HNO3 in the nitrating mixture used for nitration of benzene?
View SolutionA hydrocarbon 'A' (C4H8) on reaction HCl gives a compound 'B', (C4H9Cl) which on reaction with 1 mol of NH3 gives compounds 'C' (C4H11N). On reacting with NaNO2 and HCl followed by treatment with water compound 'C' yields an optically active alcohol, 'D'. Ozonolysis of 'A' given 2mols of acetyldehyde. Identify compound 'A' to 'D'. Explain the reaction involved.
View SolutionAn alkene 'A' (molecular formula C5H10) on ozonolysis gives a mixture of two compounds 'B' and 'C'. Compound 'B' gives positive Fehling's test and also forms iodoform on treatement with I2 and NaOH. Compound 'C' does not give Fehling's test but forms iodoform. Identify the compounds A, B and C. Write the reaction for ozonolysis and formation of iodoform from B and C.
View SolutionWrite the reactions of D-glucose which can't be explained by its open chain structure. How can cyclic structure of glucose explain these reactions?
View SolutionWhich of the following are correct reactions ?
View SolutionWhich of the following are correct reactions ?
View SolutionWhich of the following reactions is/are correct ?
View SolutionWhich of the following not reactions are correct ?
View SolutionWhich of the following reaction is/ are correct?
View SolutionWhich of the following reactions is//are correct?
View SolutionWhich of the following reactions is//are correct?
View SolutionWhich of the following reaction is/are correct?
View SolutionWhich of the following is/are correct reaction?
View SolutionWhich of the following are correct reactions ?
View SolutionWhich of the following reaction is/are correct
View SolutionWhich of the following reaction is/are correct:
View Solution

