(a) Arrange the following compounds in the increasing order of their acid strength:
p-creson, p-nitrophenol, phenol ltbr> (b) Write the mechanism (using curved arrow notation) of the following reaction:
CH2=CH2H3O+−−−→CH3−CH+2+H2O
(a) p-cresolltphenolltp-nitrophenol
(b) 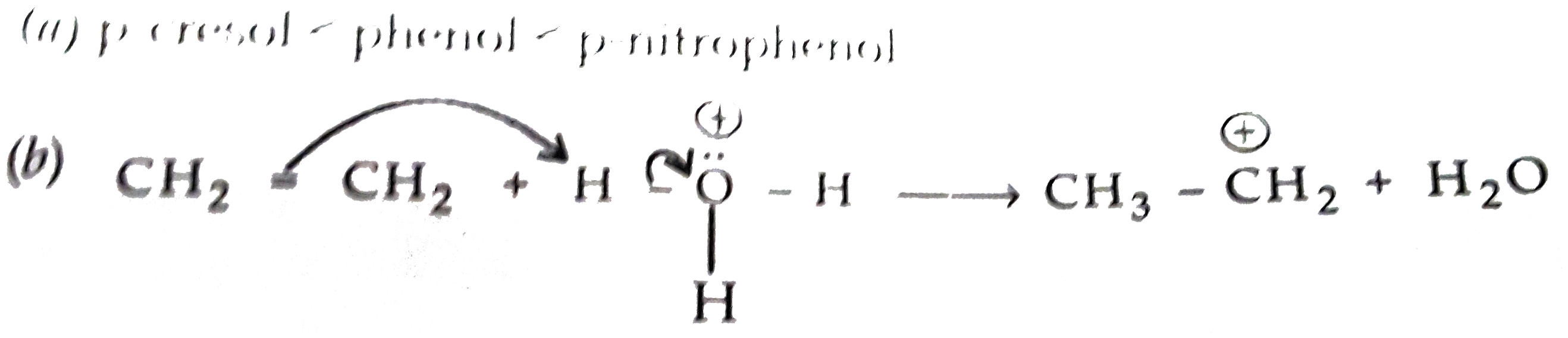
Topper's Solved these Questions
- XII BOARDSBook:XII BOARD PREVIOUS YEAR PAPER ENGLISHChapter:XII BOARDSExercise:SET-II
Explore 32 Videos
- XII BOARDSBook:XII BOARD PREVIOUS YEAR PAPER ENGLISHChapter:XII BOARDSExercise:SET-III
Explore 31 Videos
- SAMPLE PAPER 2019Book:XII BOARD PREVIOUS YEAR PAPER ENGLISHChapter:SAMPLE PAPER 2019Exercise:SECTION: D
Explore 1 Video
Similar Questions
Write the structure of 2-aminotoluene.
View SolutionWhat is especially observed when a beam of light is passed through a colloidal solution?
View Solution(a) The e.m.f. of the following cell at 298 K is 0.1745 V
Fe(s)/Fe2+(0.1M)//H+(xM)/H2(g)(1 bar)/Pt(s)
Given : E0Fe2+/Fe=−0.44V
Calculate the H+ ions concentration of the solution at the electrode where hydrogen is being produced.
(b) Aqueous solution of copper sulphate and silver nitrate are electrolysed by 1 ampere current for 10 minutes in separate electrolytic cells. Will the mass of copper and silver deposited on the cathode be same or different? Explain your answer.
View SolutionArrange the following in the increasing order of acidic strength
(I)H2CO
(II) CH3CHO
(III) C6H5CH2CHO
(IV)CH3−O∣∣C−CH2−O∣∣C−CH3
View SolutionRank the transition states that occur during the following reaction steps in order of increasing stability (least → stable)
1. H3C−+OH2→CH+3+H2O
2. (CH3)3C−+OH2→(CH3)3C++H2O
3. (CH3)2CH−+OH2→(CH3)2CH++H2O
View SolutionRank the transition states that occur during the following reaction steps in order of increasing stability (least rarr most stable): {:(1.,H_(3)C-overset(+)(O)H_(2)toCH_(3)^(+)+H_(2)O),(2.,(CH_(3))_(3)C-overset(+)(O)H_(2)to(CH_(3))_(3)C^(+)+H_(2)O),(3.,(CH_(3))_(2)CH-overset(+)(O)H_(2)to(CH_(3))_(2)CH^(+)+H_(2)O):}
View SolutionAn organic compound on reaction with O3 followed by Zn and H2Ogives CH3−O∣∣C−CH2−CH2−O∣∣C−CH3 . The structures are:
View SolutionArrange in decreasing order of acidic strengths , H2O,CH3OH,(CH3)2CHOH.
View SolutionWrite the mechanism (using curved arrow notation) for the following reaction
CH2=CH2H2O+−−−→CH3−CH2+H2O
View SolutionWrite mechanism of the following reaction: CH_(3)CH_(2)Ohoverset(HBr)toCH_(3)CH_(2)Br+H_(2)O .
View SolutionWrite the mechanism of the following reaction
CH3CH2OHHBr−−→CH3CH2Br+H2O.
View Solution(a) Arrange the following compounds in the increasing order of their acid strength: p-creson, p-nitrophenol, phenol ltbr> (b) Write the mechanism (using curved arrow notation) of the following reaction: CH_(2)=CH_(2)overset(H_(3)O^(+))toCH_(3)-CH_(2)^(+)+H_(2)O
View Solution(a) Write the formula of reagents used in the following reactions: (i) Bromination of phenol to 2, 4,6-tribromophenol (ii) Hydroboration of propene and then oxidation to propanol. (b) Arrange the following compound groups in the increasing order of their property indicated. (i) p-nitrophenol, ethanol, phenol (acidic character) (ii) Propanol, Propane, Propanal (boiling point) (c) Write the mechanism (using curved arrow notation) of the following reaction: CH_(3) - CH_(2) - overset(+)(O)H_(2) overset(CH_(3) CH_(2) OH)rarr CH_(3) - CH_(2) - underset(H)underset(|)overset(+)(O) - CH_(2) - CH_(3) + H_(2)O
View Solution(A)Write the mechanism of the following raction :
CH3CH2OHHBr−−→CH3CH2Br+H2O
(b) Write the equation involved in Reimer-Tiemann reaction.
View Solutionनिम्नलिखित अभिक्रिया की (घुमावदार तीर अंकन का उपयोग करते हुए) क्रियाविधि लिखिए-
CH2=CH2=H3O+−−−→CH3−CH+2+H2O
View SolutionWrite the mechanism of the following reaction: CH_(3)CH_(2)OH overset(HBr)(rarr)CH_(3)CH_(2)Br+H_(2)O
View Solution
