Two moles of organic compounds 'A' on treatment with a strong base gives two compounds 'B' and 'C'. Compound 'B' on dehydrogention with Cu gives 'A' while acidification of 'C' yields carboxylic and 'D' with molecular formula of CH2O2. Identify the compound A, B, C, and D.
To solve the problem, we will identify the compounds A, B, C, and D step by step based on the information provided.
Step 1: Identify Compound A
The problem states that two moles of organic compound A react with a strong base to produce compounds B and C. Based on the context, we can deduce that compound A is likely a simple aldehyde or alcohol.
Solution:
- A = Formaldehyde (HCHO)
Hint: Consider common organic compounds that can react with strong bases to form alcohols and salts.
Step 2: Identify Compound B
The reaction of compound A with a strong base produces two compounds, one of which is B. Since we are treating formaldehyde with a strong base, it can lead to the formation of an alcohol.
Solution:
- B = Methanol (CH₃OH)
Hint: Think about the products formed when aldehydes react with strong bases.
Step 3: Identify Compound C
The other product formed from the reaction of compound A with the strong base is compound C. Given that B is methanol, we can infer that C is likely a salt formed from the reaction of formaldehyde with the base.
Solution:
- C = Sodium formate (HCOONa) or the corresponding salt.
Hint: Look for the salt formed from the reaction of an aldehyde with a strong base.
Step 4: Identify Compound D
The problem states that upon acidification of compound C, a carboxylic acid and compound D are produced. Since C is sodium formate, acidification will yield formic acid (HCOOH) and liberate sodium chloride.
Solution:
- D = Formic acid (HCOOH)
Hint: Consider the products formed when a salt of a carboxylic acid is treated with an acid.
Summary of Compounds
- A = Formaldehyde (HCHO)
- B = Methanol (CH₃OH)
- C = Sodium formate (HCOONa)
- D = Formic acid (HCOOH)
Final Answer
- A: Formaldehyde (HCHO)
- B: Methanol (CH₃OH)
- C: Sodium formate (HCOONa)
- D: Formic acid (HCOOH)
The problem states that two moles of organic compound A react with a strong base to produce compounds B and C. Based on the context, we can deduce that compound A is likely a simple aldehyde or alcohol.
- A = Formaldehyde (HCHO)
The reaction of compound A with a strong base produces two compounds, one of which is B. Since we are treating formaldehyde with a strong base, it can lead to the formation of an alcohol.
- B = Methanol (CH₃OH)
The other product formed from the reaction of compound A with the strong base is compound C. Given that B is methanol, we can infer that C is likely a salt formed from the reaction of formaldehyde with the base.
- C = Sodium formate (HCOONa) or the corresponding salt.
The problem states that upon acidification of compound C, a carboxylic acid and compound D are produced. Since C is sodium formate, acidification will yield formic acid (HCOOH) and liberate sodium chloride.
- D = Formic acid (HCOOH)
- A = Formaldehyde (HCHO)
- B = Methanol (CH₃OH)
- C = Sodium formate (HCOONa)
- D = Formic acid (HCOOH)
- A: Formaldehyde (HCHO)
- B: Methanol (CH₃OH)
- C: Sodium formate (HCOONa)
- D: Formic acid (HCOOH)
Similar Questions
Identify the compounds A, B, C, D, E and F.
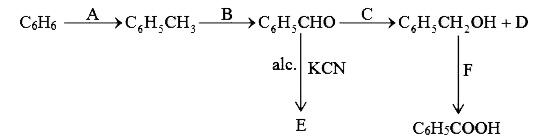
View SolutionIdentify the compounds A, B, C, D, E and F:∗∗
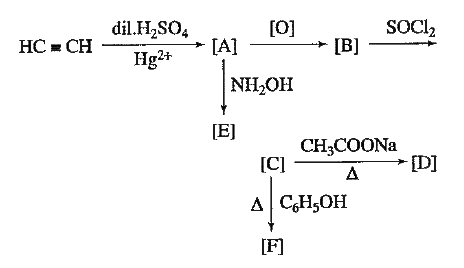
View SolutionA compound 'A' when reacts with LiAlH4 it gives two compounds B and C along with LiCl. Compounds A, B and C respectively are
View SolutionAn organic compound (A) on treatment with ethyl alcohol gives a carboxylic acid (B) and compound (C). The hydrolysis of (C) under acidic conditions gives (B) and (D). Oxidation of (D) with KMnO4 also gives (B). (B) on heating with Ca(OH)2 gives (E) (molecular formula, C3H6O). (E) does not give Tollens test and does not reduce Fehling's solution but forms a 2,4−dinitrophenyl hydrazone. Identify (A),(B),(C),(D), and (E).
View SolutionAn organic compound (A) on treatment with ethyl alcohol gives a carboxylic acid (B) and compound (C). The hydrolysis of (C) under acidic conditions gives (B) and (D). Oxidation of (D) with KMnO4 also gives (B). (B) on heating with Ca(OH)2 gives (E) (molecular formula, C3H6O). (E) does not give Tollens test and does not reduce Fehling's solution but forms a 2,4−dinitrophenyl hydrazone. Identify (A),(B),(C),(D), and (E).
View SolutionAn organic compound (A) on treatment with ethyl alcohol gives a carboxylic acid (B) and compound (C). The hydrolysis of (C) under acidic conditions gives (B) and (D). Oxidation of (D) with KMnO4 also gives (B). (B) on heating with Ca(OH)2 gives (E) (molecular formula, C3H6O). (E) does not give Tollens test and does not reduce Fehling's solution but forms a 2,4−dinitrophenyl hydrazone. Identify (A),(B),(C),(D), and (E).
View SolutionAn aromatic compound 'A' (Molecular formula C8H8O)) gives positive 2, 4-DNP test. It gives a yellow precipitate of compound 'B' on treatment with iodine and sodium hydroxide solution. Compound 'A' does not give Tollen's or Fehling's test. On drastic oxidation with potassium permanganate, it forms a carboxylic acid 'C' (Molecular formula C7H6O2), which is also formed along with the yellow compound in the above reaction. Identify A, B and C and write all the reactions involved.
View SolutionAn organic compound A’ with molecular formula C7H7NO reacts with Br2/aqKOH to give compound B’, which upon reaction with NaNO2&HCl at O∘C gives C’. Compound C’ on heating with CH3CH2OH gives a hydrocarbon D’. Compound B’ on further reaction with Br2 water gives white precipitate of compound E’. Identify the compound A, B, C, D&E, also justify your answer by giving relevant chemical equations.
View SolutionAn aromatic compound 'A' (molecular formula C8H8O) gives positive 2, 4-DNP test. It gives a yellow precipitate of compound 'B' on treatment with iodine and sodium hydroxide solution. Compound 'A' does not give Tollen's or Fehling's test. On drastic oxidation with potassium permaganate it forms a carboxylic acid 'C' (molecular formula C7H6O2), which is also formed alongwith the yellow compound in the above reaction. Identify A, B and C and write all the reactions involved.
View SolutionAn aromatic compound 'A' (molecular formula C8H8O) gives positive 2, 4-DNP test. It gives a yellow precipitate of compound 'B' on treatment with iodine and sodium hydroxide solution. Compound 'A' does not give Tollen's or Fehling's test. On drastic oxidation with potassium permaganate it forms a carboxylic acid 'C' (molecular formula C7H6O2), which is also formed alongwith the yellow compound in the above reaction. Identify A, B and C and write all the reactions involved.
View Solution

