(a) Complete the following chemical reaction equations :
(i) P4+SO2Cl2→
(ii) XeF6+H2O→
(b) Predict the shape and the asked angle (90∘ or mor or less) in each of the following cases :
(i) SO2−3 and the angle O−S−O
(ii) ClF3 and the angleF−Cl−F
(iii) XeF2 and the angle F−Xe−F
(a) (i) P4+8SO2Cl2→4PCl3+4SO2+PS2Cl2
(ii) XeF6+H2O→2HF+XeOF4
(b) (i) Structure :
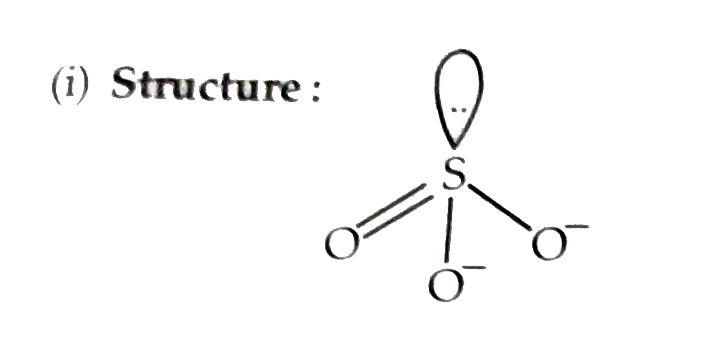
Shapeof SO32− pyramidal.
Bond angle → More than 180∘.
(ii) Strucutre:
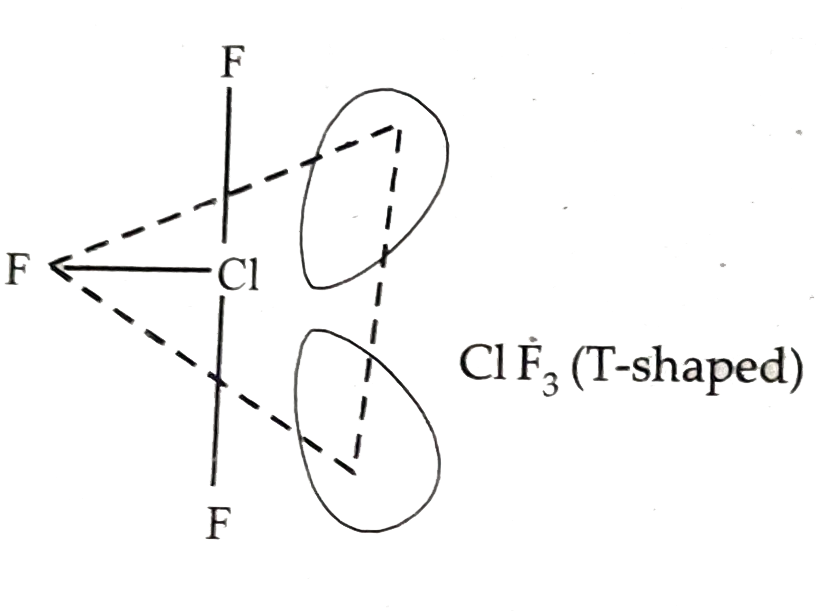
ClF3→ Bent T - shape Bond angle = More than 90∘.
(iii) Structure
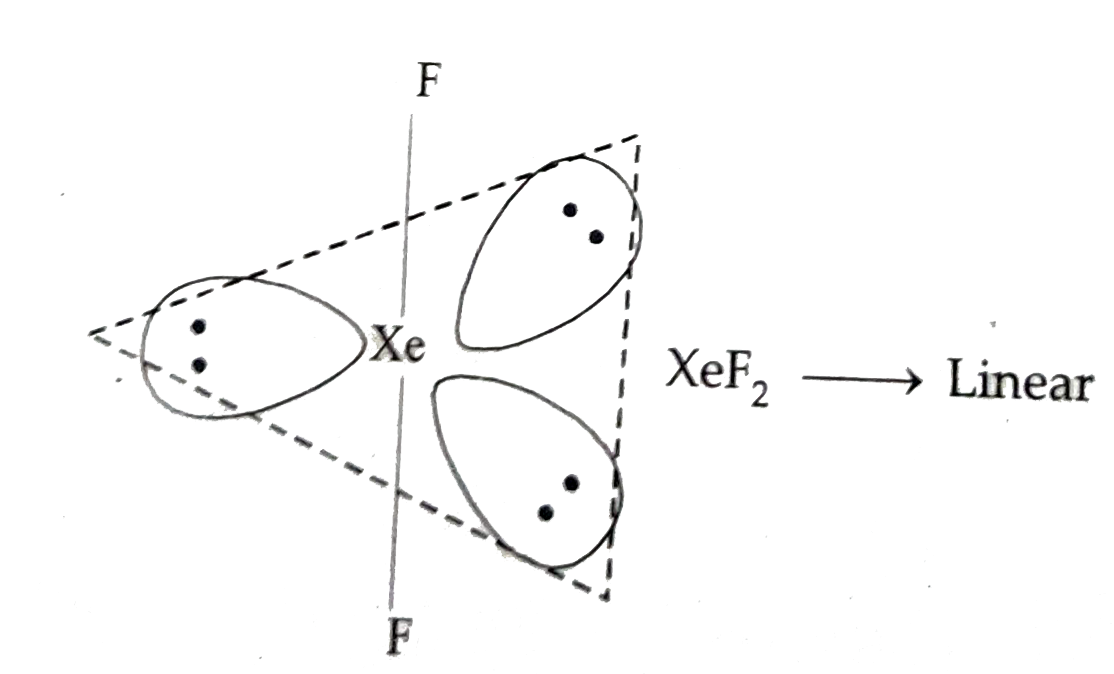
XeF2→ Linear Shape Bond angle = More than 180∘.
Topper's Solved these Questions
- XII BOARDSBook:XII BOARD PREVIOUS YEAR PAPER ENGLISHChapter:XII BOARDSExercise:SET-II
Explore 32 Videos
- XII BOARDSBook:XII BOARD PREVIOUS YEAR PAPER ENGLISHChapter:XII BOARDSExercise:SET-III
Explore 31 Videos
- SAMPLE PAPER 2019Book:XII BOARD PREVIOUS YEAR PAPER ENGLISHChapter:SAMPLE PAPER 2019Exercise:SECTION: D
Explore 1 Video
Similar Questions
Complete the following chemical equations :
XeF2+H2O→
View SolutionComplete the following chemical equations :
XeF6(s)+H2O(l)→
View SolutionComplete the following chemical equation :
XeF4+H2O→
View SolutionComplete the following chemical equations :
(i) NaOH+Cl2→(hot and conc.)
(ii) XeF4+O2F2→
View SolutionComplete the following chemical equations :
(i) SO2+MnO−4+H2O→
(ii) F2(g)+H2O(l)→
View SolutionComplete the following chemical equations :
(i) P4+ SOCl2 →
(ii)F2(Excess)+Cl2300∘C−−−→
View SolutionComplete the following chemical reaction equations :
(i) XeF2+H2O→
(ii) PH3+HgCl2→
View SolutionComplete the following chemical equation :
(i) P4+NaOH+H2O→
(ii)XeF4+O2F2→
View SolutionComplete the following chemical equations :
(i) HgCl2+PH3→(ii)SO3+H2SO4→
(iii) XeF4+H2O→
View SolutionComplete the following chemical reaction equations :
(i)KClO3Heat−−−→MnO2
(ii) XeF4+H2O→
View Solution
