If water sample are taken from sea, rivers or lake, they will be found to contain hydrogen and oxygen in the approximate ratio of 1:8. This indicates the law of :
To solve the question regarding the ratio of hydrogen and oxygen in water samples from various sources, we will follow these steps:
1. Understanding the Composition of Water:
- Water (H₂O) is composed of hydrogen and oxygen. The molecular formula indicates that for every 2 atoms of hydrogen, there is 1 atom of oxygen.
2. Determining the Mass Ratio:
- The atomic mass of hydrogen (H) is approximately 1 g/mol, and the atomic mass of oxygen (O) is approximately 16 g/mol.
- Therefore, the mass of 2 grams of hydrogen corresponds to 2 grams (since there are 2 hydrogen atoms) and the mass of 1 oxygen atom is 16 grams.
3. Calculating the Total Mass of Water:
- The total mass of water formed from 2 grams of hydrogen and 16 grams of oxygen is:
Total mass of water=mass of H+mass of O=2 g+16 g=18 g
4. Finding the Ratio of Hydrogen to Oxygen:
- The ratio of the mass of hydrogen to the mass of oxygen in water is:
Ratio=mass of Hmass of O=2 g16 g=18
5. Identifying the Law:
- The consistent ratio of hydrogen to oxygen (1:8) in water, regardless of the source (sea, river, lake), indicates that the composition of a compound is always the same. This is a demonstration of the Law of Definite Proportions.
6. Conclusion:
- Therefore, the law indicated by the consistent ratio of hydrogen and oxygen in water samples from different sources is the Law of Definite Proportions.
- Water (H₂O) is composed of hydrogen and oxygen. The molecular formula indicates that for every 2 atoms of hydrogen, there is 1 atom of oxygen.
- The atomic mass of hydrogen (H) is approximately 1 g/mol, and the atomic mass of oxygen (O) is approximately 16 g/mol.
- Therefore, the mass of 2 grams of hydrogen corresponds to 2 grams (since there are 2 hydrogen atoms) and the mass of 1 oxygen atom is 16 grams.
- The total mass of water formed from 2 grams of hydrogen and 16 grams of oxygen is:
Total mass of water=mass of H+mass of O=2 g+16 g=18 g
- The ratio of the mass of hydrogen to the mass of oxygen in water is:
Ratio=mass of Hmass of O=2 g16 g=18
- The consistent ratio of hydrogen to oxygen (1:8) in water, regardless of the source (sea, river, lake), indicates that the composition of a compound is always the same. This is a demonstration of the Law of Definite Proportions.
- Therefore, the law indicated by the consistent ratio of hydrogen and oxygen in water samples from different sources is the Law of Definite Proportions.
Similar Questions
All samples of carbon dioxide contain carbon and oxygen in the mass ratio of 3:8 This is in agreement with the law of .
View SolutionStatement-I : Pure water obtained from different sources such as, river, well, spring, sea etc. always contains hydrogen and oxygen combined in the ratio 1:8 by mass.
Because
Statement-II : A chemical compound always contains elements combined together in same proportion by mass. it wa discovered by French chemist, Joseph Proust (1799).
View SolutionAssertion (A): pure water obtained from different states of india always contains hydrogen and oxygen in the ration of 1:8 by mass.
Reason (R ): Total mass of reactants and products during chemical change is always the same.
View SolutionA radio istoper X with a half-life 1.4×108 yr decays of Y which is stable. A sample of the rock from a cave was tound to contain X and Y in the ratio 1:7. The age of the rock is
View SolutionA radioactive isotope X with half-life of 693×109 years decay to Y which is stable. A sample of rock from of the moon was found to contain both the elements X Y in the mole ratio 1:7 . What is the age of the rock ?
View SolutionAt 1000K, water vapour at 1 atm has been found to be dissociated into hydrogen and oxygen to the extent of 3×10−5%. The std free energy change of the system at standard state, assuming ideal behaviour in kJ will be
View SolutionThis law was given by, a French chemist , Joseph Proust. He stated that a given compound always contains exactly the same proportion of elements by weight.
Proust worked with two samples of curpic carbonate- one of which was of natural origin and the other was synthetic one . He found that the composition of elements present in it was for both the samples as shown below:
Thus, irrespective of the source, a given compound always contains same elements in the same proportion. The validity of this law has been confrmed by various experiments. It is sometimes also reffered to as Law of constant composition.
Limitation:
This law is not applicable if the compound is formed from different isotopes of an element . The two isotopes of carbon C-12 and C-14 form carbondioxide C12O2 and C14O2 . The ratio of C : O is 12 : 32 and 14 : 32 respectively .It is not a constant ratio.
6.488 g lead with 1.002 g oxygen to form an oxide. This oxide is also obtained by heating Pb(NO3)2, It is found that % of lead in this oxide is 86.62 .Show that these date illustrate the law of definite proportions.
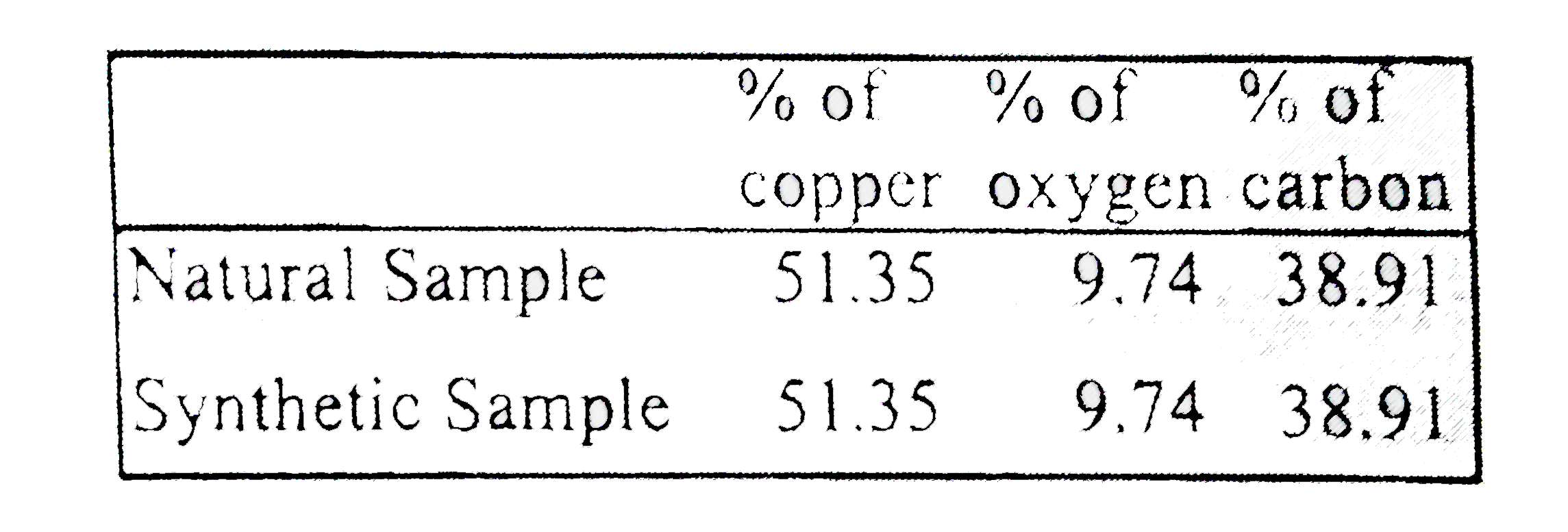
View SolutionState the term : (Do not give examples)
The substance that can remove hydrogen and oxygen atoms in the ratio of 2:1 (in the form of water) from the compounds.
View SolutionA radio isotope X with a half life 1.4×109 yr decays of Y which is stable. A sample of the rock from a cave was found to contain X and Y in the ratio 1:7. The age of the rock is
View SolutionA radio isotope X with a half life 1.4×109 yr decays of Y which is stable. A sample of the rock from a cave was found to contain X and Y in the ratio 1:7. The age of the rock is
View Solution
