A spinel is an important class of oxide consisting two types of metal ions with the oxide ions arranged in ccp layers . The normal spinel has one -eight of the tetrahedral holes occupied by one type of metal ions and one- half of the octahedral holes occupied by another type of metal ion. Such a spine is formed by Mg2+,Al3+andO2− . The neutrality of the crystal is being maintained.
Type of hole occupied by Al3+ ions is:
(a) tetrahedral
(b) octahedral
(c) both (a) and (b)
(d) None of these
The correct Answer is:B
To determine the type of hole occupied by Al³⁺ ions in the spinel structure formed by Mg²⁺, Al³⁺, and O²⁻, we will follow these steps:
Step 1: Understand the Structure of Spinel
Spinel structures are characterized by the arrangement of oxide ions in a cubic close-packed (CCP) lattice. In this structure:
- The number of atoms per unit cell (Z) is 4.
- The number of tetrahedral voids is 8 (2 times the number of atoms).
- The number of octahedral voids is 4 (equal to the number of atoms).
Step 2: Identify the Occupancy of Voids
According to the problem:
- One-eighth of the tetrahedral voids are occupied by one type of metal ion (let's assume this is Mg²⁺).
- One-half of the octahedral voids are occupied by another type of metal ion (assumed to be Al³⁺).
Step 3: Calculate the Number of Ions Occupying the Voids
- Tetrahedral Voids: Total = 8. If 1/8 of these are occupied by Mg²⁺, then:
\text{Number of Mg²⁺ ions} = \frac{1}{8} \times 8 = 1
- Octahedral Voids: Total = 4. If 1/2 of these are occupied by Al³⁺, then:
\text{Number of Al³⁺ ions} = \frac{1}{2} \times 4 = 2
Step 4: Charge Neutrality
To maintain charge neutrality in the spinel structure:
- The total negative charge from O²⁻ ions is:
Total negative charge=4 (O²⁻ ions)×2=8
- The total positive charge must also equal +8. If we assume:
- 1 Mg²⁺ contributes +2
- 2 Al³⁺ contribute 2×3=6
Thus, the total positive charge is:
Total positive charge=2+6=8
Step 5: Conclusion
Since Al³⁺ occupies the octahedral voids, the type of hole occupied by Al³⁺ ions is octahedral.
Final Answer
The type of hole occupied by Al³⁺ ions is octahedral.
---
Spinel structures are characterized by the arrangement of oxide ions in a cubic close-packed (CCP) lattice. In this structure:
- The number of atoms per unit cell (Z) is 4.
- The number of tetrahedral voids is 8 (2 times the number of atoms).
- The number of octahedral voids is 4 (equal to the number of atoms).
According to the problem:
- One-eighth of the tetrahedral voids are occupied by one type of metal ion (let's assume this is Mg²⁺).
- One-half of the octahedral voids are occupied by another type of metal ion (assumed to be Al³⁺).
- Tetrahedral Voids: Total = 8. If 1/8 of these are occupied by Mg²⁺, then:
\text{Number of Mg²⁺ ions} = \frac{1}{8} \times 8 = 1
- Octahedral Voids: Total = 4. If 1/2 of these are occupied by Al³⁺, then:
\text{Number of Al³⁺ ions} = \frac{1}{2} \times 4 = 2
To maintain charge neutrality in the spinel structure:
- The total negative charge from O²⁻ ions is:
Total negative charge=4 (O²⁻ ions)×2=8
- The total positive charge must also equal +8. If we assume:
- 1 Mg²⁺ contributes +2
- 2 Al³⁺ contribute 2×3=6
Total positive charge=2+6=8
Since Al³⁺ occupies the octahedral voids, the type of hole occupied by Al³⁺ ions is octahedral.
The type of hole occupied by Al³⁺ ions is octahedral.
Topper's Solved these Questions
- SOLID STATEBOOK - NARENDRA AWASTHI ENGLISHCHAPTER - SOLID STATEEXERCISE - Passage-4
3 Videos
- SOLID STATEBOOK - NARENDRA AWASTHI ENGLISHCHAPTER - SOLID STATEEXERCISE - Passage-5
3 Videos
- SOLID STATEBOOK - NARENDRA AWASTHI ENGLISHCHAPTER - SOLID STATEEXERCISE - Passge-2
4 Videos
- IONIC EEQUILIBRIUMBOOK - NARENDRA AWASTHI ENGLISHCHAPTER - IONIC EEQUILIBRIUMEXERCISE - Subjective problems
1 Video
- STOICHIOMETRYBOOK - NARENDRA AWASTHI ENGLISHCHAPTER - STOICHIOMETRYEXERCISE - Match the Colum-II
6 Videos
Similar Questions
Ionic lattic has two major points defects ,(1) Schottky (2) Frenkel defects . Schottkly defects occurs due to the cations - anion pair's missing from the lattice sites . Frenkel defects occurs when cation leaves its lattice site and fits into an interstitial space. The neutrality of the crystal is being maintained and we considered all losses from interstitial positions.
which defect decrease density of the crystal ?
View SolutionDoping mens introduction of small amount of impurities like phosphorus , arsenic or boron into the pure crystal . In pure silicon , ther are four valenices used in bonding with other four adjacent silicon crystal is doped with a group -15 element ( with five valence electron ) such as P, As , or Bi , the structure of the crystal lattic remains unchanged . Out of the five valence electron of group -15 doped element four element are used in normal covalent bonding with silicon while fiffth electron is delcoasiled and thus conducts electricity
Doping a silicon crystal with a group -13 element (with three valence electrons ) such as B, Al, Ga or In products a semiconductor with three electrons in in dopant . The place where fourth electron is missing is called an electron vacancy or hole . Such hole can move throught the crystal like a positive charge giving rise conduction of electricity.
No. of valene electrons in silicon are :
View Solutionpacking fraction of a unit cell is drfined as the fraction of the total volume of the unit cell occupied by the atom(s).
P.E=Volume of the atoms(s) present in a unit cellVolume of unit cell=Z×43πr3a3
and % of empty space = 100−P.F.×100
where Z= effective number of stoms in s cube .
r= radius of a an atoms
a = edge lenght of the cube
Packing fraction in face centered cubic unit cell is :
View SolutionCalculate pOH of 0.1 M aq. Solution of weak base BOH (Kb=10−7) at 25∘C.
View Solution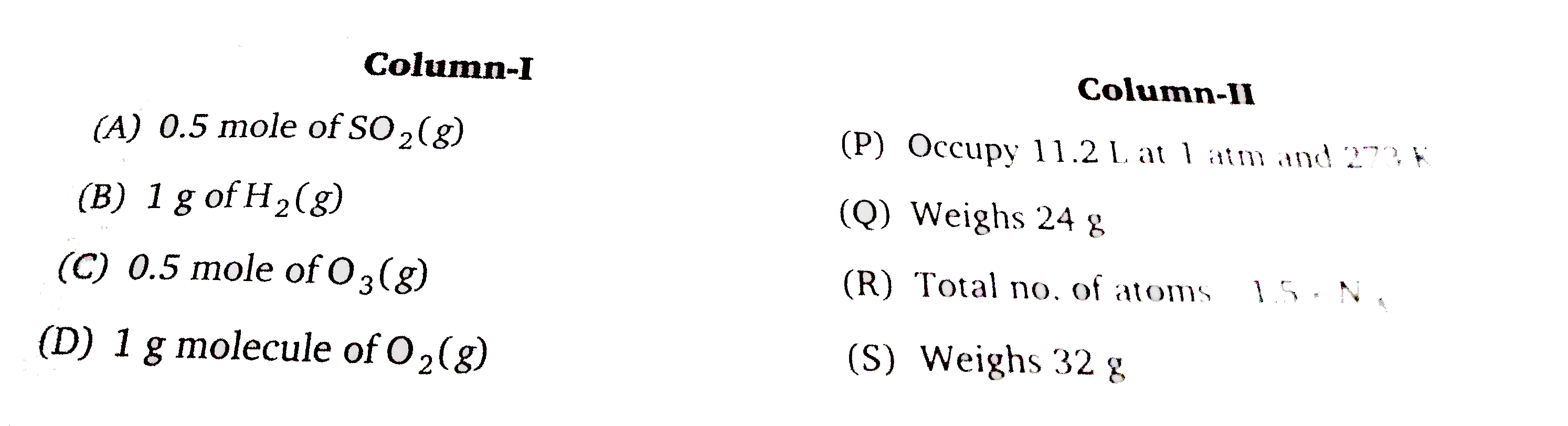
View SolutionA spinel is an important class of oxide consisting two types of metal ions withs the oxide ions arranged in ccp layers . The normal spinel has one -eight of the tetrahendral holes occupied by one type of metal ions and one- half of the octaherdral holes occupied by another type of metal ion. Such a spine is formed by Mg2+,Al3+andO2− . The netutrality of the crystal is benig maintained.
The fromule of the spinel is :
View SolutionA spinel is an important class of oxide consisting two types of metal ions withs the oxide ions arranged in ccp layers . The normal spinel has one -eight of the tetrahendral holes occupied by one type of metal ions and one- half of the octaherdral holes occupied by another type of metal ion. Such a spine is formed by Mg2+,Al3+andO2− . The netutrality of the crystal is benig maintained.
Type of hole occupied by Al3+ ions is:
View SolutionA spinel is an important class of oxide consisting two types of metal ions withs the oxide ions arranged in ccp layers . The normal spinel has one -eight of the tetrahendral holes occupied by one type of metal ions and one- half of the octaherdral holes occupied by another type of metal ion. Such a spine is formed by Mg2+,Al3+andO2− . The netutrality of the crystal is benig maintained.
Type of hole occupied by Mg2+ ions is:
View SolutionA spinel is an important class of oxide consisting two types of metal ions withs the oxide ions arranged in ccp layers . The normal spinel has one -eight of the tetrahendral holes occupied by one type of metal ions and one- half of the octaherdral holes occupied by another type of metal ion. Such a spine is formed by Mg^(2+), Al^(3+) and O^(2-) . The netutrality of the crystal is benig maintained. If oxide ion is replaced by X^(-8//3) , the number of anionic vacancy per unit cells is :
View SolutionA spinal is an important class of oxides consisting of two types of metal ions with oxides ions arranged in CCP layer. The normal spinal has one-eighth of the tetrahedral holes occupied by one type of metal ion and one-half of the octahedral holes occupied by another type of metal ion. such a spinal is formed by Zn2+,Al3+andO2− in the tetrahedral holes. if formula of the compound is ZnxAlyOz, then find the value of (x+y+z) ? The type of packing generated by type (I) is:
View SolutionIn a normal spinel types structure, the oxide ions are arranged in ccp whereas 1/8 tetrahedral holes are occupied by Zn^(2+) ions and 50% of octahedral holes are occupied by Fe^(3+) ions. The formula of the compound is -
View SolutionSpinel is a important class of oxides consisting of two types of metal ions with the oxide ions arranged in CCP pattern. The normal spinel has one-eight of the tetrahedral holes occupied by one type of metal ion and one half of the octahedral hole occupied by another type of metal ion. Such a spinel is formed by Zn^(2+) , Al^(3+) and O^(2–) , with Zn^(2+) in the tetrahedral holes. Give the formulae of spinel.
View SolutionSpinel is a important class of oxides consisting of two types of metal ions with the oxide ions arranged in CCP pattern. The normal spinel has one-eight of the tetrahedral holes occupied by one type of metal ion and one half of the octahedral hole occupied by another type of metal ion. Such a spinel is formed by Zn2+, Al3+ and O2–, with Zn2+ in the tetrahedral holes. Give the formulae of spinel.
View SolutionA spinel is an important class of oxides consising of two types of metal ions with the oxide ions arranged in cop layers. The normal spinel has one-eigth of the tetrahedral holes occupied by one type of metal ion and one-half of the octahedral holes occupied by another type of metal ion. Such a spinel is formed by Mg^(2+), A^(2+) and O^(2-) . The neutratrality of the crystal is maintained. The formula of the spinel is
View SolutionA spinel is an important class of oxides consising of two types of metal ions with the oxide ions arranged in cop layers. The normal spinel has one-eigth of the tetrahedral holes occupied by one type of metal ion and one-half of the octahedral holes occupied by another type of metal ion. Such a spinel is formed by Mg^(2+), A^(2+) and O^(2-) . The neutratrality of the crystal is maintained. Type of hole occupied by Al^(3+) ions is :
View Solution

