Select the correct statement (s) :
(a) The co-ordinaton number of each type of ions a CsCl crystal is 12
(b) A metal that crystallizes in a bcc structure has a co-ordination number of 12
(c) A unit cell of an ionic crystal shares some of its ions with other units cells
(d) The length of the unit cell in NaCl in 552 "pm" (given that r+Na= 85 "pm" and r−Cl= 181 " pm")
The correct Answer is:C
To solve the question, we need to analyze each statement regarding the properties of ionic crystals, particularly focusing on the CsCl and NaCl structures. Let's go through each statement step by step.
1. Statement 1: Coordination number of each type of ions in a CsCl crystal is 12.
- In a CsCl crystal, the chloride ions (Cl⁻) are located at the corners of the cube, and the cesium ions (Cs⁺) are located at the body center.
- The coordination number refers to the number of nearest neighbors surrounding an ion. In CsCl, each Cs⁺ ion is surrounded by 8 Cl⁻ ions and vice versa.
- Therefore, the coordination number for both Cs⁺ and Cl⁻ is 8, not 12.
- Conclusion: This statement is incorrect.
2. Statement 2: A metal that crystallizes in a BCC structure has a coordination number of 12.
- In a body-centered cubic (BCC) structure, there are atoms at the corners and one atom at the body center.
- The coordination number for a BCC structure is also 8, as each atom at the body center is surrounded by 8 corner atoms.
- Conclusion: This statement is incorrect.
3. Statement 3: A unit cell of an ionic crystal shares some of its ions with other unit cells.
- This statement is true. In ionic crystals, the unit cell does share ions with adjacent unit cells. For example, the ions at the edges and corners of the unit cell are shared with neighboring unit cells.
- Conclusion: This statement is correct.
4. Statement 4: Length of the unit cell in NaCl is 552 picometers given that sodium ion is 85 picometers and chloride ion is 181 picometers.
- In NaCl, the chloride ions are arranged in a face-centered cubic (FCC) structure, and sodium ions occupy the octahedral voids.
- The edge length of the NaCl unit cell can be calculated as follows:
- The edge length a is given by the formula:
a=2(RCl+RNa)
- Substituting the values:
a=2(181 pm+85 pm)=2(266 pm)=532 pm
- Since the given value is 552 picometers, which is not equal to the calculated value of 532 picometers, this statement is also incorrect.
Final Conclusion:
- The only correct statement is Statement 3.
- In a CsCl crystal, the chloride ions (Cl⁻) are located at the corners of the cube, and the cesium ions (Cs⁺) are located at the body center.
- The coordination number refers to the number of nearest neighbors surrounding an ion. In CsCl, each Cs⁺ ion is surrounded by 8 Cl⁻ ions and vice versa.
- Therefore, the coordination number for both Cs⁺ and Cl⁻ is 8, not 12.
- Conclusion: This statement is incorrect.
- In a body-centered cubic (BCC) structure, there are atoms at the corners and one atom at the body center.
- The coordination number for a BCC structure is also 8, as each atom at the body center is surrounded by 8 corner atoms.
- Conclusion: This statement is incorrect.
- This statement is true. In ionic crystals, the unit cell does share ions with adjacent unit cells. For example, the ions at the edges and corners of the unit cell are shared with neighboring unit cells.
- Conclusion: This statement is correct.
- In NaCl, the chloride ions are arranged in a face-centered cubic (FCC) structure, and sodium ions occupy the octahedral voids.
- The edge length of the NaCl unit cell can be calculated as follows:
- The edge length a is given by the formula:
a=2(RCl+RNa)
- Substituting the values:
a=2(181 pm+85 pm)=2(266 pm)=532 pm
- Since the given value is 552 picometers, which is not equal to the calculated value of 532 picometers, this statement is also incorrect.
- The only correct statement is Statement 3.
Topper's Solved these Questions
- SOLID STATEBOOK - NARENDRA AWASTHI ENGLISHCHAPTER - SOLID STATEEXERCISE - Match the column
10 Videos
- SOLID STATEBOOK - NARENDRA AWASTHI ENGLISHCHAPTER - SOLID STATEEXERCISE - Assertion - Reason type Question
11 Videos
- SOLID STATEBOOK - NARENDRA AWASTHI ENGLISHCHAPTER - SOLID STATEEXERCISE - Passage-7
3 Videos
- IONIC EEQUILIBRIUMBOOK - NARENDRA AWASTHI ENGLISHCHAPTER - IONIC EEQUILIBRIUMEXERCISE - Subjective problems
1 Video
- STOICHIOMETRYBOOK - NARENDRA AWASTHI ENGLISHCHAPTER - STOICHIOMETRYEXERCISE - Match the Colum-II
6 Videos
Similar Questions
Match Column-I to II
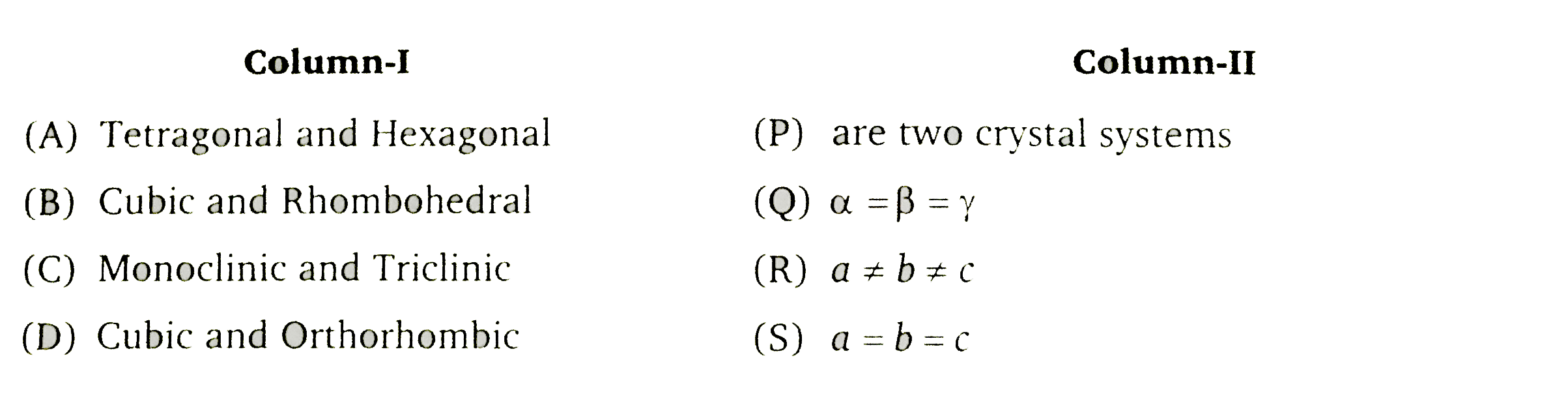
View SolutionStatement I: In any ionic solid [MX] with Schottky defect, the number of positive and negative ions are same.
Statement II: An equal number of cation and anion vacancies is present.
View SolutionIn diamond structure ,carbon atoms form fcc lattic and 50% tetrahedral voids occupied by acrbon atoms . Evergy carbon atoms is surrounded tetrachedral by four carbon atom with bond length 154 pm . Germanium , silicon and grey tin also crystallise in same way as diamond (NA=6×1023)
The mass of diamond unit cell is:
View SolutionCalculate pOH of 0.1 M aq. Solution of weak base BOH (Kb=10−7) at 25∘C.
View Solution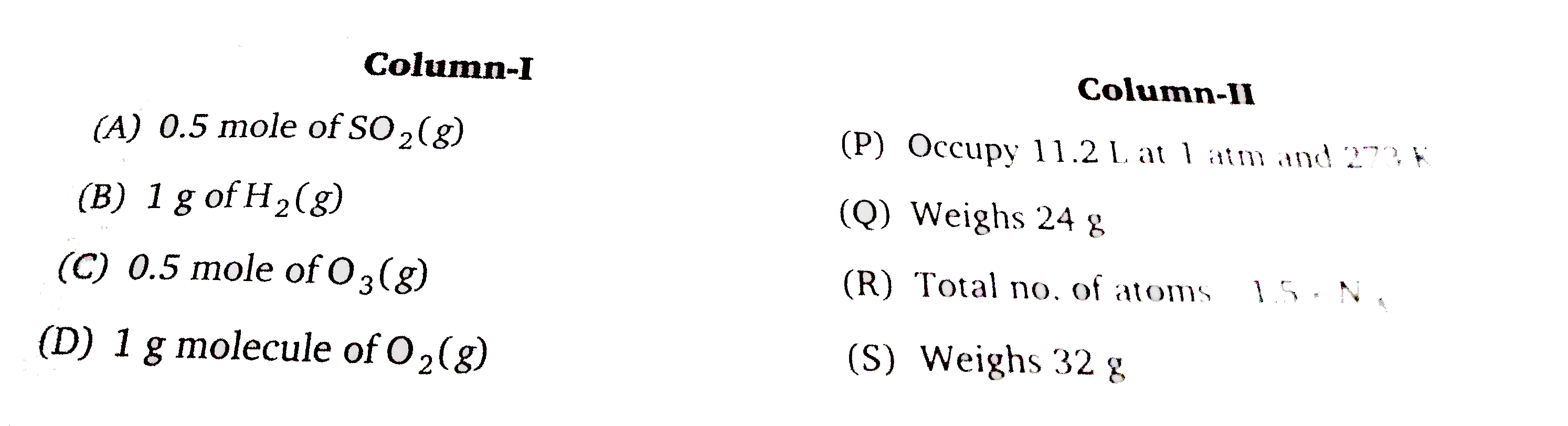
View SolutionSelect the correct statement (s).
View SolutionSelect the correct statement (s).
View SolutionSelect the correct statement(s):
View SolutionSelect the correct statement(s):
View SolutionSelect the correct statement(s):
View SolutionSelect the correct statement(s):
View SolutionSelect the correct statement(s):
View SolutionSelect the correct statement(s):
View SolutionSelect the correct statement(s):
View SolutionSelect the correct statements (s) :
View SolutionSelect the correct statements/s:
View Solution

