Which state of the triply ionized Beryllium (Be3+) has the same orbit radius as that of the ground state of hydrogen atom?
3
2
4
5
The correct Answer is:B
To solve the problem of determining which state of the triply ionized Beryllium (Be3+) has the same orbit radius as that of the ground state of a hydrogen atom, we will use Bohr's model of the atom.
1. Understand the Bohr Model Formula:
The radius of an electron in a hydrogen-like atom is given by the formula:
\(
r = a_0 \frac{n^2}{Z}
\)
where:
- \( r \) is the radius of the orbit,
- \( a_0 \) is the Bohr radius (approximately \( 0.529 \, \text{Å} \)),
- \( n \) is the principal quantum number (orbit number),
- \( Z \) is the atomic number of the element.
2. Identify Parameters for Hydrogen:
For the hydrogen atom:
- \( Z = 1 \) (since hydrogen has one proton),
- The ground state corresponds to \( n = 1 \).
Thus, the radius of the ground state of hydrogen is:
\(
r_H = a_0 \frac{1^2}{1} = a_0
\)
3. Identify Parameters for Triply Ionized Beryllium:
For triply ionized Beryllium (Be3+):
- The atomic number \( Z = 4 \) (Beryllium has four protons),
- We need to find the value of \( n \) such that the radius of the orbit equals that of hydrogen.
4. Set Up the Equation for Beryllium:
We want the radius of the orbit in Beryllium to equal that of hydrogen:
\(
r_{Be^{3+}} = a_0 \frac{n^2}{Z} = a_0
\)
Substituting \( Z = 4 \):
\(
a_0 \frac{n^2}{4} = a_0
\)
5. Solve for \( n^2 \):
Dividing both sides by \( a_0 \) (assuming \( a_0 \neq 0 \)):
\(
\frac{n^2}{4} = 1
\)
Multiplying both sides by 4:
\(
n^2 = 4
\)
6. Find \( n \):
Taking the square root of both sides:
\(
n = 2
\)
Conclusion:
The state of the triply ionized Beryllium (Be3+) that has the same orbit radius as that of the ground state of hydrogen atom is when \( n = 2 \), which corresponds to the first excited state.
The radius of an electron in a hydrogen-like atom is given by the formula:
\(
r = a_0 \frac{n^2}{Z}
\)
where:
- \( r \) is the radius of the orbit,
- \( a_0 \) is the Bohr radius (approximately \( 0.529 \, \text{Å} \)),
- \( n \) is the principal quantum number (orbit number),
- \( Z \) is the atomic number of the element.
For the hydrogen atom:
- \( Z = 1 \) (since hydrogen has one proton),
- The ground state corresponds to \( n = 1 \).
\(
r_H = a_0 \frac{1^2}{1} = a_0
\)
For triply ionized Beryllium (Be3+):
- The atomic number \( Z = 4 \) (Beryllium has four protons),
- We need to find the value of \( n \) such that the radius of the orbit equals that of hydrogen.
We want the radius of the orbit in Beryllium to equal that of hydrogen:
\(
r_{Be^{3+}} = a_0 \frac{n^2}{Z} = a_0
\)
Substituting \( Z = 4 \):
\(
a_0 \frac{n^2}{4} = a_0
\)
Dividing both sides by \( a_0 \) (assuming \( a_0 \neq 0 \)):
\(
\frac{n^2}{4} = 1
\)
Multiplying both sides by 4:
\(
n^2 = 4
\)
Taking the square root of both sides:
\(
n = 2
\)
The state of the triply ionized Beryllium (Be3+) that has the same orbit radius as that of the ground state of hydrogen atom is when \( n = 2 \), which corresponds to the first excited state.
Topper's Solved these Questions
- ATOMIC STUCTUREBOOK - NARENDRA AWASTHI ENGLISHCHAPTER - ATOMIC STUCTUREEXERCISE - level 2
30 Videos
- ATOMIC STUCTUREBOOK - NARENDRA AWASTHI ENGLISHCHAPTER - ATOMIC STUCTUREEXERCISE - Level 3 (Passage 1)
3 Videos
- CHEMICAL EQUILIBRIUMBOOK - NARENDRA AWASTHI ENGLISHCHAPTER - CHEMICAL EQUILIBRIUMEXERCISE - Match the column
1 Video
Similar Questions
Which energy state of triply ionized beryllium (Be+3) has the same orbital radius as that of state of hydrogen atom
View SolutionWhat is the energy of state in a triply ionized beryllium (Be+++) whose radius is equal to that of ground state of hydrogen ?
View SolutionWhich energy state of doubly ionized lithium Li++ has the same energy as that of the ground state of hydrogen?
View SolutionWhich enrgy state of doubly ionized lithium (Li++ has the same energy as that of the gorund state of hydrogen?
View SolutionHydrogen atom:
The electronic ground state of hydrogen atom contains one electron in the first orbit. If sufficient energy is provided, this electron can be promoted to higher energy levels. The electronic energy of a hydrogen-like species (any atom//ions with nuclear charge Z and one electron) can be given as
En=−RHZ2n2 where RH=Rydberg constant,n=principal quantum number
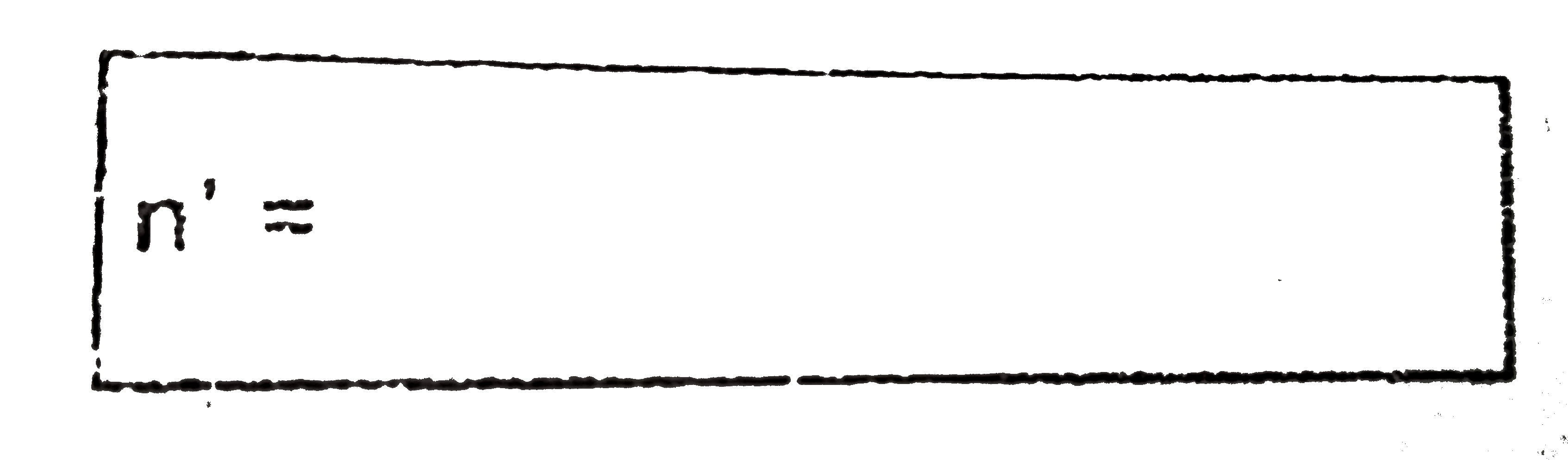
What is the principal quantum number, n' of the orbit of Be3 that has the same Bohr radius as that of ground state hydrogen atom ?
View SolutionFind out the wavelength of the electron orbiting in the ground state of hydrogen atoms.
View SolutionThe radius of which of the following orbit is same as that of the first Bohr's orbit of hydrogen atom.
View SolutionWhen a hydrogen atom is raised the ground state to third state
View SolutionThe ratio of areas within the elctron orbits for the first excited state to the ground sate for hydrogen atom is
View SolutionThe excited energy of a He+ ion is the same as the ground state energy of hydrogen is it always true that one of the energies of any hydrogen like ion will be the same as the ground state energy of a hydrogen atom?
View Solution

