For an equilibrium H2O(s)⇔H2O(l), which of the following statements is ture ?
The pressure changes do not affect the equilibrium
More of ice melts if pressure on the system is increased
More of liquid freezes if prssure on the system is increased
Less of ice melts if the pressure on the system is increased.
The correct Answer is:b
To determine which statement is true regarding the equilibrium H2O(s)⇌H2O(l), we will analyze each statement based on the principles of chemical equilibrium and the effects of pressure on phase changes.
1. Understanding the Equilibrium:
The equilibrium H2O(s)⇌H2O(l) represents the phase change of ice (solid water) melting into liquid water.
2. Effect of Pressure on Equilibrium:
According to Le Chatelier's principle, if an external change is applied to a system at equilibrium, the system will adjust to counteract that change. In the case of phase changes involving solids and liquids, pressure can significantly affect the equilibrium position.
3. Analyzing the Statements:
- Statement 1: "The pressure changes do not affect the equilibrium."
- This statement is false. Pressure changes do affect the equilibrium, especially in systems involving solids and liquids.
- Statement 2: "More of ice melts if the pressure on the system is increased."
- This statement is true. Increasing pressure favors the formation of the phase with a smaller volume. Since liquid water has a smaller volume than ice, increasing pressure will shift the equilibrium to the right, resulting in more ice melting into liquid water.
- Statement 3: "More of the liquid freezes if pressure on the system is increased."
- This statement is false. As established, increasing pressure favors melting rather than freezing in this equilibrium.
- Statement 4: "Less of ice melts if the pressure on the system is increased."
- This statement is false. As explained, increasing pressure leads to more ice melting, not less.
4. Conclusion:
The only true statement regarding the equilibrium H2O(s)⇌H2O(l) is Statement 2: "More of ice melts if the pressure on the system is increased."
Summary:
The correct answer is Statement 2.
The equilibrium H2O(s)⇌H2O(l) represents the phase change of ice (solid water) melting into liquid water.
According to Le Chatelier's principle, if an external change is applied to a system at equilibrium, the system will adjust to counteract that change. In the case of phase changes involving solids and liquids, pressure can significantly affect the equilibrium position.
- Statement 1: "The pressure changes do not affect the equilibrium."
- This statement is false. Pressure changes do affect the equilibrium, especially in systems involving solids and liquids.
- Statement 2: "More of ice melts if the pressure on the system is increased."
- This statement is true. Increasing pressure favors the formation of the phase with a smaller volume. Since liquid water has a smaller volume than ice, increasing pressure will shift the equilibrium to the right, resulting in more ice melting into liquid water.
- Statement 3: "More of the liquid freezes if pressure on the system is increased."
- This statement is false. As established, increasing pressure favors melting rather than freezing in this equilibrium.
- Statement 4: "Less of ice melts if the pressure on the system is increased."
- This statement is false. As explained, increasing pressure leads to more ice melting, not less.
The only true statement regarding the equilibrium H2O(s)⇌H2O(l) is Statement 2: "More of ice melts if the pressure on the system is increased."
The correct answer is Statement 2.
Similar Questions
For the equlibrium H2O(s)⇔H2O(l) which of the following statements is true?
View SolutionWhich of the following statements about H2O2 is not true ?
View SolutionFor a physical equilibrium, H2 O (Ice) ⇔H2 O (Water ) which of the following is the true statement:
View Solution
(C) H3O+/H2O−−−−−−−→(E)
(D) H3O+/H2O−−−−−−−→ (F)
Which of the following statements is / are correct ?
View SolutionFor the equilibrium
H2O(1)⇔H2O(g)
at 1 atm 298K
View Solution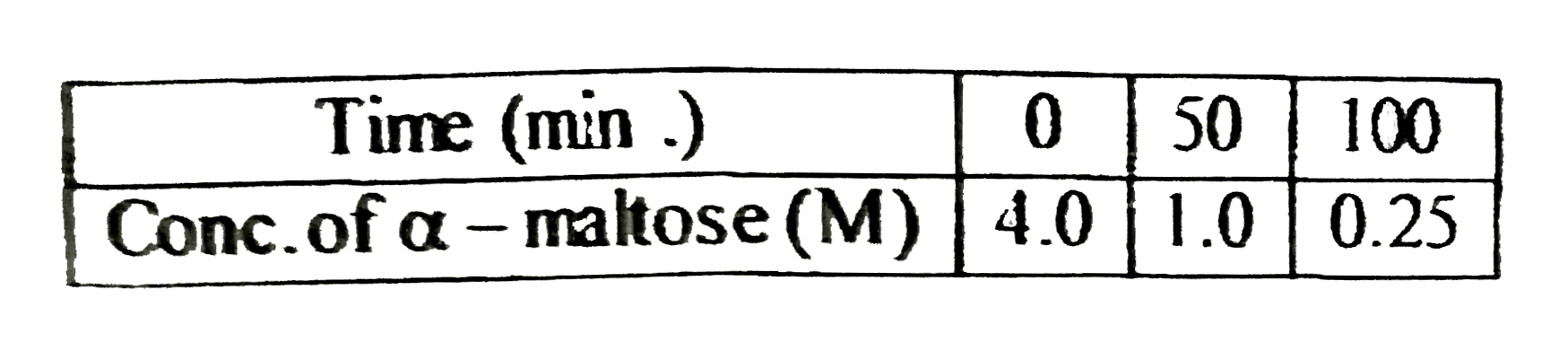
α- maltose (C12H22O11) can be hydrolysed to glucose (C6H12O6) according to the following reaction.
C12H22(O11(aq)+H2O(l)→2C6H12O6(aq)
Given:
Standard enthalpy of formation of C12H22O11(aq)=−2238kJmol
Standard enthalpy of formation of H2O(l)=−285kJmol
Standard enthalpy of formation of C6H12O6(aq)=−1263kJmol
Which of the following statements (s) is/are true?
View SolutionFor a process H2O(s)→H2O(l)at273K
View SolutionConsider equilibrium H2O(l)⇔H2O(g). Choose the correct direction of shifting of equilibrium with relative humidity.
View SolutionHg2l2⏐⏐ ⏐ ⏐↓(green)boiled−−−−→withH2O products
Which of the following statement is correct with respect to the products ?
View SolutionDecreasing in the pressure for the following equilibria: H2O(s)⇔H2O(l) result in the :
View Solution
