Azeotropic mixture of water and C2H5OH boils at 351 K. By distilling the mixture it is possible to obtain
pure C2H5OH only
Pure water only
NeitherC2H5OH nor water
Both water and C2H5OH in pure state
The correct Answer is:c
To solve the problem regarding the azeotropic mixture of water and ethanol (C₂H₅OH) that boils at 351 K, we need to understand the nature of azeotropes and what can be obtained from distillation.
1. Understanding Azeotropes:
- An azeotropic mixture is a mixture of two or more liquids that has a constant boiling point and composition throughout the distillation process. This means that when the mixture is boiled, the vapor produced has the same composition as the liquid mixture.
2. Composition of the Mixture:
- In the case of the azeotropic mixture of water and ethanol, the boiling point is given as 351 K. At this temperature, the mixture will not separate into pure components upon boiling.
3. Distillation Process:
- During distillation of an azeotropic mixture, the vapor that forms will have the same ratio of components as the liquid mixture. Therefore, no matter how much you distill, you will not be able to obtain pure ethanol or pure water.
4. Conclusion:
- Since the question asks what can be obtained from distilling the azeotropic mixture, the answer is that it is not possible to obtain pure ethanol or pure water. The composition remains constant, and you will continue to get a mixture of water and ethanol.
Final Answer:
By distilling the azeotropic mixture of water and ethanol that boils at 351 K, it is not possible to obtain pure ethanol or pure water. The distillation will yield a mixture with the same composition as the original azeotropic mixture.
- An azeotropic mixture is a mixture of two or more liquids that has a constant boiling point and composition throughout the distillation process. This means that when the mixture is boiled, the vapor produced has the same composition as the liquid mixture.
- In the case of the azeotropic mixture of water and ethanol, the boiling point is given as 351 K. At this temperature, the mixture will not separate into pure components upon boiling.
- During distillation of an azeotropic mixture, the vapor that forms will have the same ratio of components as the liquid mixture. Therefore, no matter how much you distill, you will not be able to obtain pure ethanol or pure water.
- Since the question asks what can be obtained from distilling the azeotropic mixture, the answer is that it is not possible to obtain pure ethanol or pure water. The composition remains constant, and you will continue to get a mixture of water and ethanol.
By distilling the azeotropic mixture of water and ethanol that boils at 351 K, it is not possible to obtain pure ethanol or pure water. The distillation will yield a mixture with the same composition as the original azeotropic mixture.
Topper's Solved these Questions
- DILUTE SOLUTIONBOOK - NARENDRA AWASTHI ENGLISHCHAPTER - DILUTE SOLUTIONEXERCISE - leval-02
26 Videos
- DILUTE SOLUTIONBOOK - NARENDRA AWASTHI ENGLISHCHAPTER - DILUTE SOLUTIONEXERCISE - leval-03
23 Videos
- CHEMICAL EQUILIBRIUMBOOK - NARENDRA AWASTHI ENGLISHCHAPTER - CHEMICAL EQUILIBRIUMEXERCISE - Match the column
1 Video
- ELECTROCHEMISTRYBOOK - NARENDRA AWASTHI ENGLISHCHAPTER - ELECTROCHEMISTRYEXERCISE - Subjective problems
14 Videos
Similar Questions
Two beaker A and B present in a closed vessel. Beaker A contains 152.4 g aqueous soulution of urea, containing 12 g of urea. Beaker B contain 196.2 g glucose solution, containing 18 g of glocose. Both solution allowed to attain the equilibrium. Determine mass % of glocose in its solution at equilibrium allowed to attain the equilibrium :
View SolutionLowering in vapour pressure is determined by Ostwald and Walker dynamic methed. It is based on the prinicipal , that when air is allowed to pass through a solvent or solution, it takes up solventvapour with it to get itself saturated at that temperature
I and II are weighted separately before and after passing dry air. Loss in mass of each set, gives the lowing of vapour pressure. The temperature of air, the solution and the solvent is kept constant.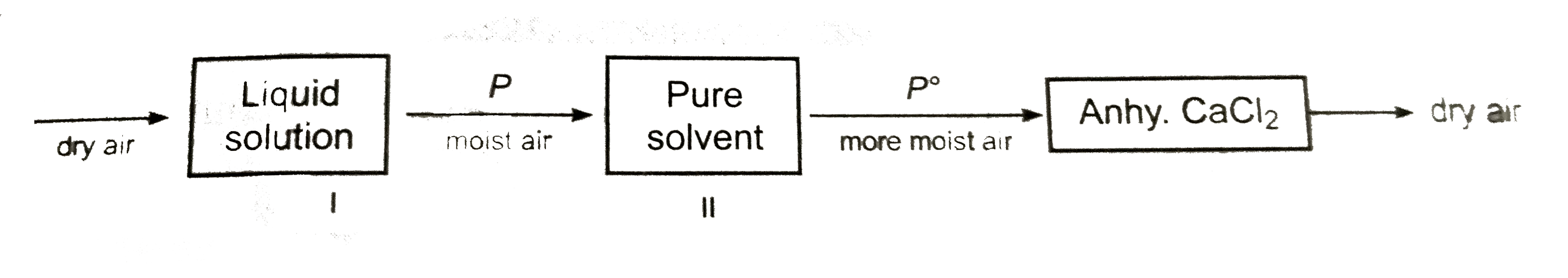
Loss in masss of solvent (wII)will be proportional to :
View SolutionColumn-I and Column-II contains fore enteries each. Entries of Column-I are to be matched with, some entries of Column-II One or more than one entries of Column-I may have the mathching with the same entries of Column-II
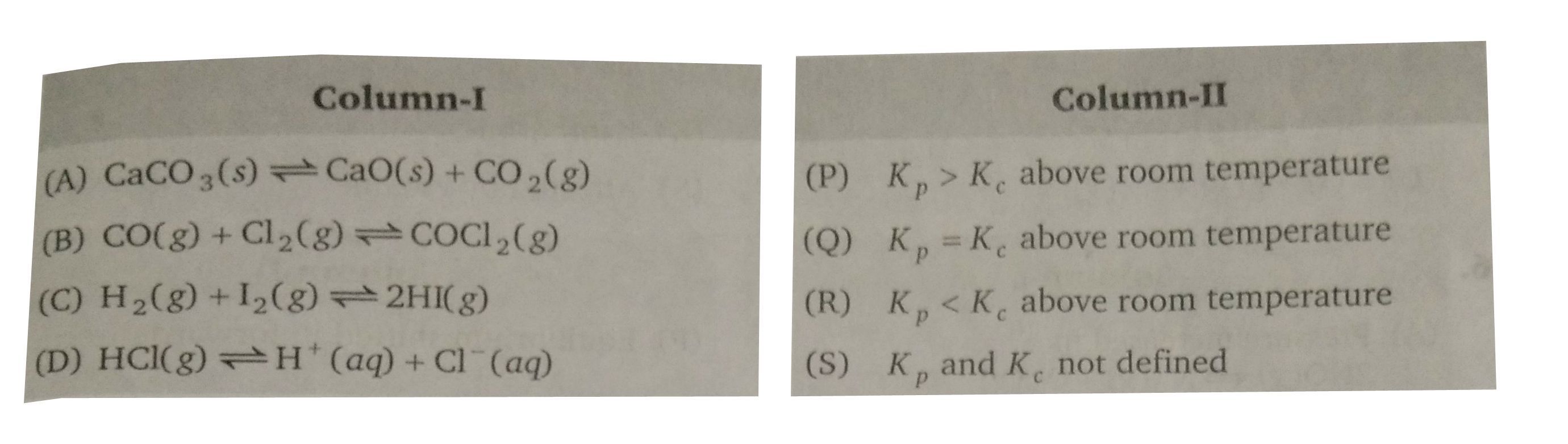
View SolutionHow many faradays are required for reduction of 1mol C6H5NO2 into C6H5NH2?
View SolutionAssertion : Ethanol and water mixture can be separed by simple distillation.
Reason : Ethanol and water forms azeotropic mixture.
View SolutionPure water boils at 373.15 K and nitric acid boils at 359.15 K . An azeotropic mixture of H_(2)O and HNO_(3) boils at 393.15 K . Distilling the azeotropic mixture will cause
View SolutionThe azeotropic mixture of water and HCl boils at 110∘C when this mixture is simple distilled it is possible to obtain
View SolutionThe azeotropic mixture of water (B.P.=100∘C) and HCl(B.P.=86∘C)boils at about 120∘C. During fractional distillation of this mixture it is possible to obtain :
View SolutionAzeotropic mixture of water and C2H5OH boils at 351 K. By distilling the mixture it is possible to obtain
View SolutionThe azetrophic mixture of water (b.pt. 100∘C) and HCl (b.pt. 85∘C) boils at 108.5∘C. What this mixture is distilled, it is possible to obtain :
View SolutionAzeotropic mixture of water and HCl boils at 381.5 K. By distilling the mixture it is possible to obtain-
View SolutionThe azeotropic mixture of water (b.pt 100∘C) and HCl (b.pt 85∘C) boils at 108.5∘C. When this mixture is distilled, it is possible to obtain:
View SolutionThe azeotropic mixture of water (B Pt 100^@C) and HCl (B. Pt 85^@ C) boils at 108 ^@C . When this mixture is distilled it is possible to obtain :
View SolutionFractional distillation|Azeotropes mixture
View SolutionThe azeotropic mixture of water and HCl boils at 108.5^(@)C . When this mixture is distilled, it is possible to obtain
View Solution

