During a process ideal gas expands to compress the spring such that 10kJ energy is stored in the spring and 2kJ heat is released from gas to surrounding find the change in internal energy of gas
12kJ
10kJ
8kJ
6kJ
The correct Answer is:A
To solve the problem, we will use the first law of thermodynamics, which states:
ΔU=Q−W
where:
- ΔU is the change in internal energy,
- Q is the heat added to the system,
- W is the work done by the system.
1. Identify the given values:
- Energy stored in the spring (work done by the gas) = 10 kJ
- Heat released from the gas to the surroundings = 2 kJ
2. Assign the correct signs:
- Since the gas releases heat, Q will be negative:
Q=−2 kJ
- The work done by the gas (compressing the spring) is positive:
W=10 kJ
3. Substitute the values into the first law of thermodynamics:
ΔU=Q−W
Substituting the values:
ΔU=(−2 kJ)−(10 kJ)
4. Calculate the change in internal energy:
ΔU=−2 kJ−10 kJ=−12 kJ
5. Interpret the result:
The negative sign indicates that the internal energy of the gas has decreased. However, the question asks for the magnitude of the change in internal energy, which is:
|ΔU|=12 kJ
Final Answer:
The change in internal energy of the gas is 12 kJ.
---
- ΔU is the change in internal energy,
- Q is the heat added to the system,
- W is the work done by the system.
- Energy stored in the spring (work done by the gas) = 10 kJ
- Heat released from the gas to the surroundings = 2 kJ
- Since the gas releases heat, Q will be negative:
Q=−2 kJ
- The work done by the gas (compressing the spring) is positive:
W=10 kJ
ΔU=Q−W
Substituting the values:
ΔU=(−2 kJ)−(10 kJ)
ΔU=−2 kJ−10 kJ=−12 kJ
The negative sign indicates that the internal energy of the gas has decreased. However, the question asks for the magnitude of the change in internal energy, which is:
|ΔU|=12 kJ
The change in internal energy of the gas is 12 kJ.
Topper's Solved these Questions
Similar Questions
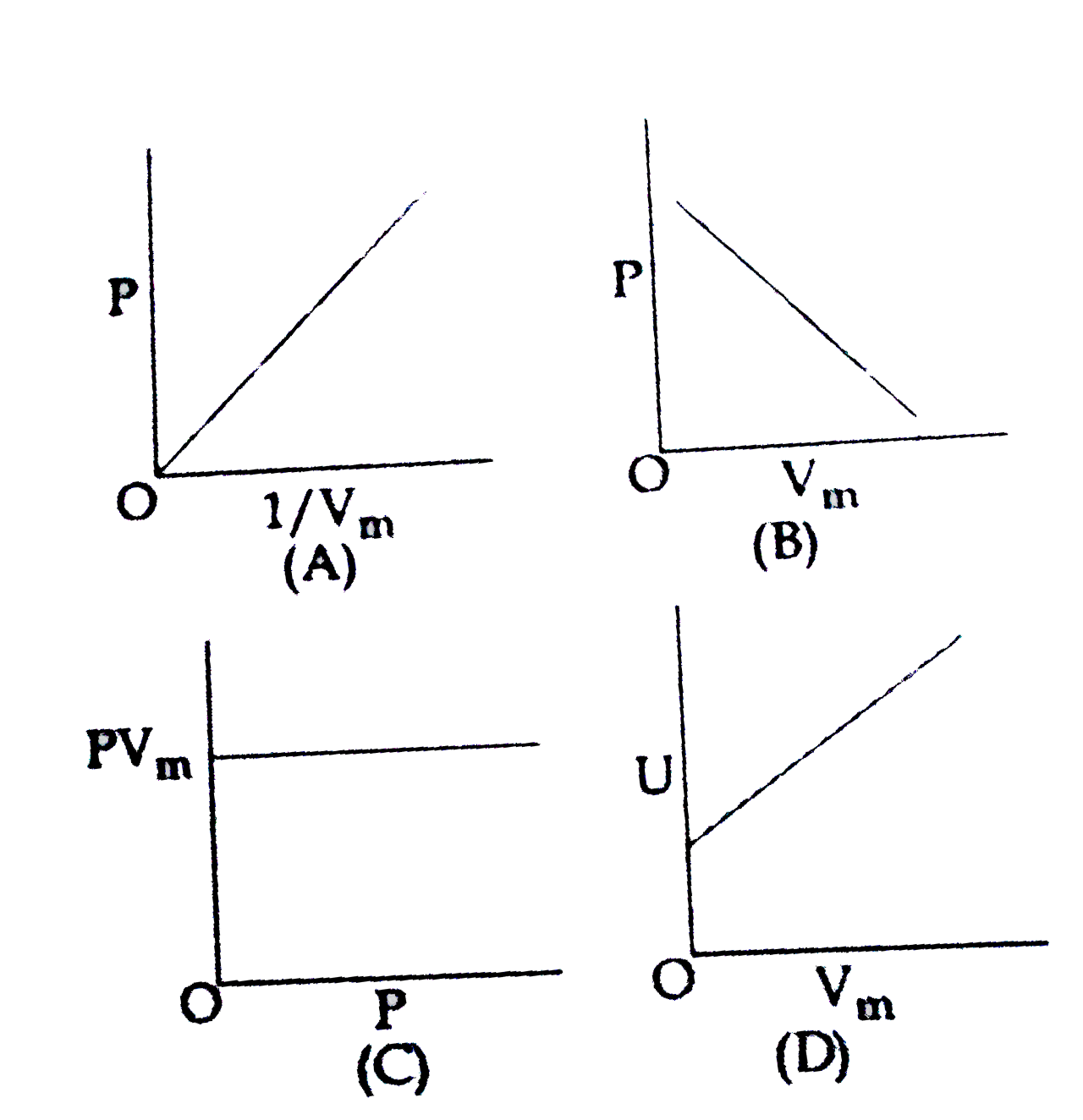
The combination of plots which does not represent isothermal expansion of an ideal gas is :
View SolutionA box weighs 196 N on a spring balance at the noth pole. Its weight recorded on the same balance if it is shifted to the equator is close to (take g=10ms−2 at the north pole and the radius of the earth = 6400 km) :
View SolutionIf x and y are arbitrary intensive variables, then
View SolutionDuring a process ideal gas expands to compress the spring such that 10kJ energy is stored in the spring and 2kJ heat is released from gas to surrounding find the change in internal energy of gas
View Solutionp-V diagram of an ideal gas for a process ABC is as shown in the figure. (a) Find total heat absorbed or released by the gas during the process ABC. (b) Change in internal energy of the gas during the process ABC. (c) Plot pressure versus density graph of the gas for the process ABC.
View SolutionAn ideal gas expand against a constant external pressure at 2.0 atmosphere from 20 litre to 40 litre and absorb 10kJ of energy from surrounding . What is the change in internal energy of the system ?
View SolutionThe internal energy of an ideal gas increases during an isothermal process when the gas is
View SolutionChange in internal energy in an isothermal process for ideal gas is
View Solutionचक्रीय प्रक्रम में आदर्श गैस की आन्तरिक ऊर्जा में परिवर्तन
View SolutionAn ideal gas expand against a constant external pressure at 2.0 atmosphere from 20 litre to 40 litre and absorb 10kJ of energy from surrounding . What is the change in internal energy of the system ?
View SolutionIn a thermodynamic process the pressure of a fixed mass of gas is changed. In this process gas releases 20J heat and 8J of work is done on the gas. If initial internal energy of the gas was 30J, then final internal energy is
View SolutionIn a thermodynamic process the pressure of a fixed mass of gas is changed. In this process gas releases 20J heat and 8J of work is done on the gas. If initial internal energy of the gas was 30J, then final internal energy is
View SolutionP-V diagram of an ideal gas for a process ABC is as shown in the figure.
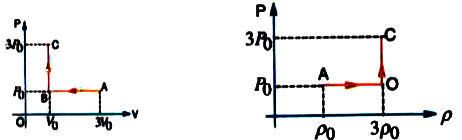
Change in internal energy of the gas during the process ABC
View Solutionअभिकथनः किसी समतापी प्रसार में, गैस ऊष्मा अवशोषित करती है तथा कार्य करती है जबकि किसी समतापी संपीडन में, पर्यावरण द्वारा गैस पर कार्य किया जाता है तथा ऊष्मा निकलती है।
तर्कः किसी समतापी प्रक्रम में, किसी आदर्श गैस की आन्तरिक ऊर्जा में कोई परिवर्तन नहीं होता है।
View Solution(A) : During an adiabatic compression temperature of an ideal gas increase (R ) : Internal energy of a gas can be changed even without addition or withdrawn of heat but by doing work
View Solution

