Which of the following pairs represents linkage isomers?
[Cu(NH3)4][PtCl4]and[Pt(NH3)4][CuCl4]
[Pb(P Ph3)2(NCS)2]and[Pb(PPh3)2(SCN)2]
[Co(NH3)5NO3]SO4and[CO(NH3)5SO4]NO3
[PtCl2(NH3)4Br2and[PtBr2(NH3)4]Cl2
The correct Answer is:B
To determine which of the given pairs represents linkage isomers, we first need to understand the concept of linkage isomerism and ambidentate ligands.
1. Understanding Linkage Isomers:
Linkage isomers are a type of isomerism that occurs in coordination compounds. They arise when a ligand can coordinate to the metal center through different atoms. This often involves ambidentate ligands, which have more than one donor atom.
2. Identifying Ambidentate Ligands:
An ambidentate ligand can bind to a metal ion through two different atoms. A common example is the thiocyanate ion (SCN⁻), which can bind through either the sulfur (S) or the nitrogen (N) atom.
3. Analyzing the Options:
We need to look at the pairs provided in the options to see if any contain ambidentate ligands.
4. Example of SCN⁻:
For instance, if we have a pair that includes (M(SCN)₂) where one is bound through sulfur and the other through nitrogen, this would represent linkage isomers.
5. Evaluating Each Option:
- Option 1: Contains ligands that do not exhibit ambidentate behavior.
- Option 2: Contains SCN⁻ as a ligand, which can bind through either sulfur or nitrogen, thus representing linkage isomers.
- Option 3: Similar to option 1, does not show linkage isomerism.
- Option 4: Again does not contain ambidentate ligands.
6. Conclusion:
The only pair that represents linkage isomers is the one that includes SCN⁻, as it can coordinate through different atoms.
Final Answer:
The correct option that represents linkage isomers is Option 2, which includes the ambidentate ligand SCN⁻.
---
Linkage isomers are a type of isomerism that occurs in coordination compounds. They arise when a ligand can coordinate to the metal center through different atoms. This often involves ambidentate ligands, which have more than one donor atom.
An ambidentate ligand can bind to a metal ion through two different atoms. A common example is the thiocyanate ion (SCN⁻), which can bind through either the sulfur (S) or the nitrogen (N) atom.
We need to look at the pairs provided in the options to see if any contain ambidentate ligands.
For instance, if we have a pair that includes (M(SCN)₂) where one is bound through sulfur and the other through nitrogen, this would represent linkage isomers.
- Option 1: Contains ligands that do not exhibit ambidentate behavior.
- Option 2: Contains SCN⁻ as a ligand, which can bind through either sulfur or nitrogen, thus representing linkage isomers.
- Option 3: Similar to option 1, does not show linkage isomerism.
- Option 4: Again does not contain ambidentate ligands.
The only pair that represents linkage isomers is the one that includes SCN⁻, as it can coordinate through different atoms.
The correct option that represents linkage isomers is Option 2, which includes the ambidentate ligand SCN⁻.
Topper's Solved these Questions
Similar Questions
Knowledge Check
Which of the following pairs represents anomers ?
A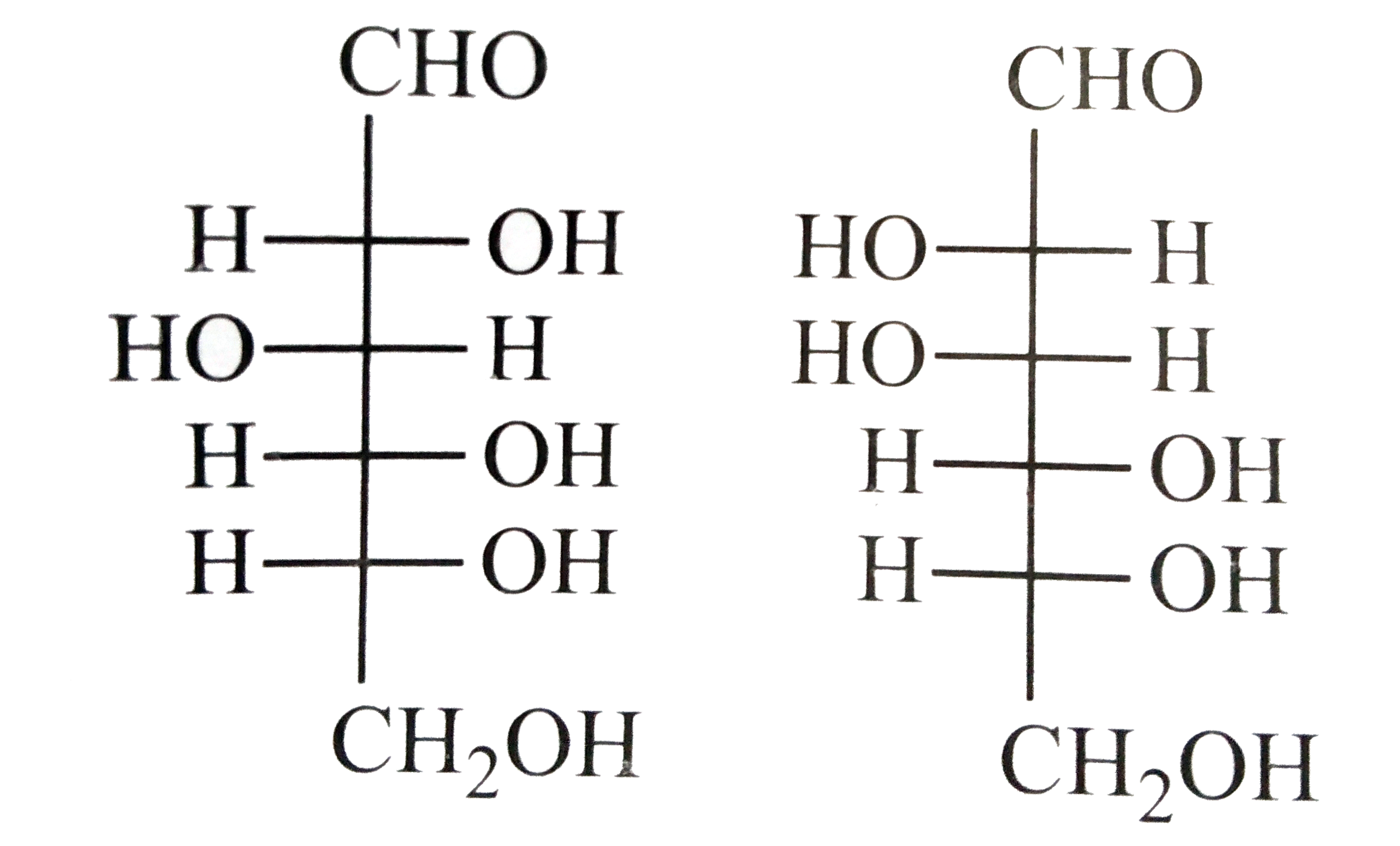 B
B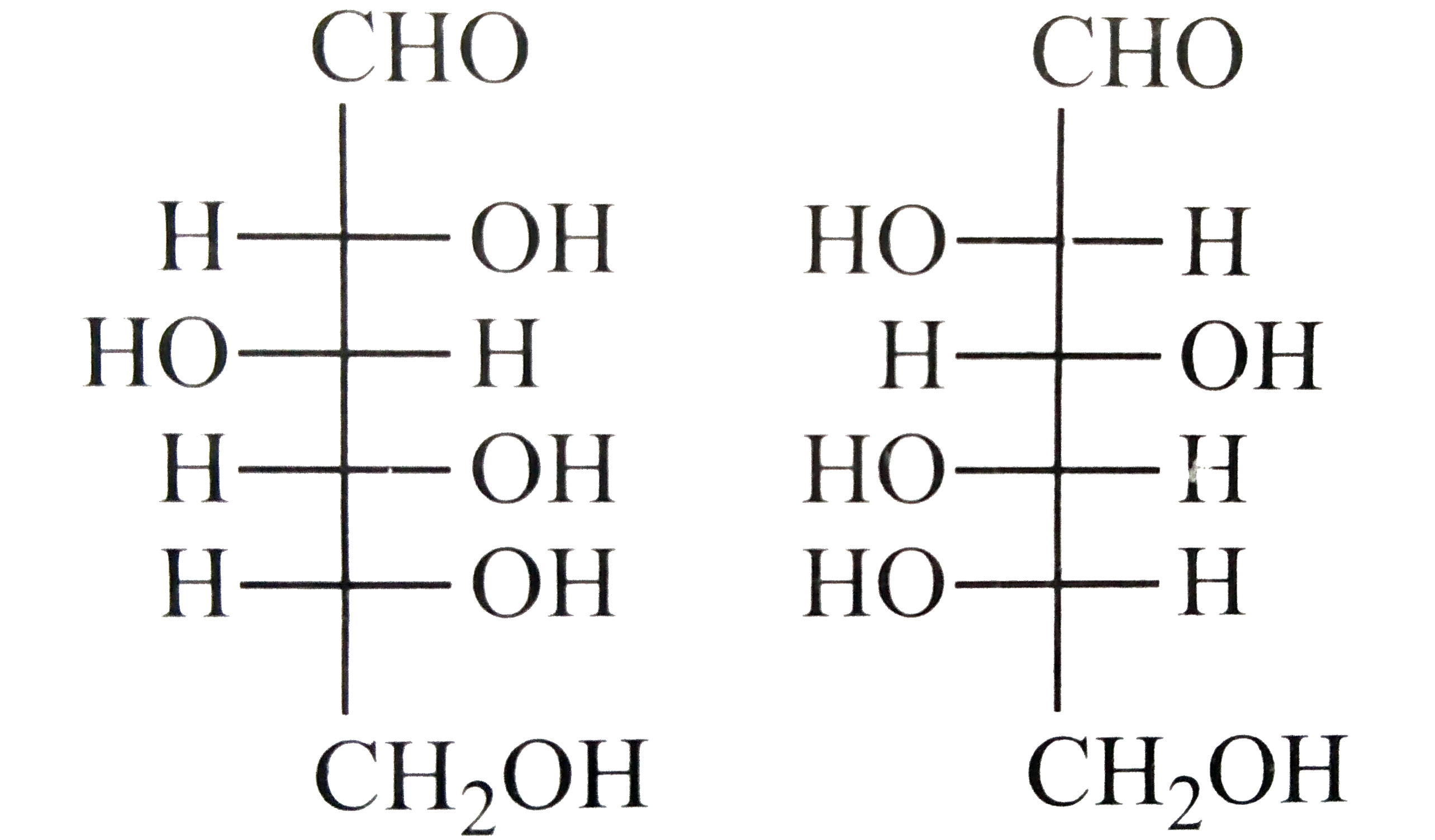 C
C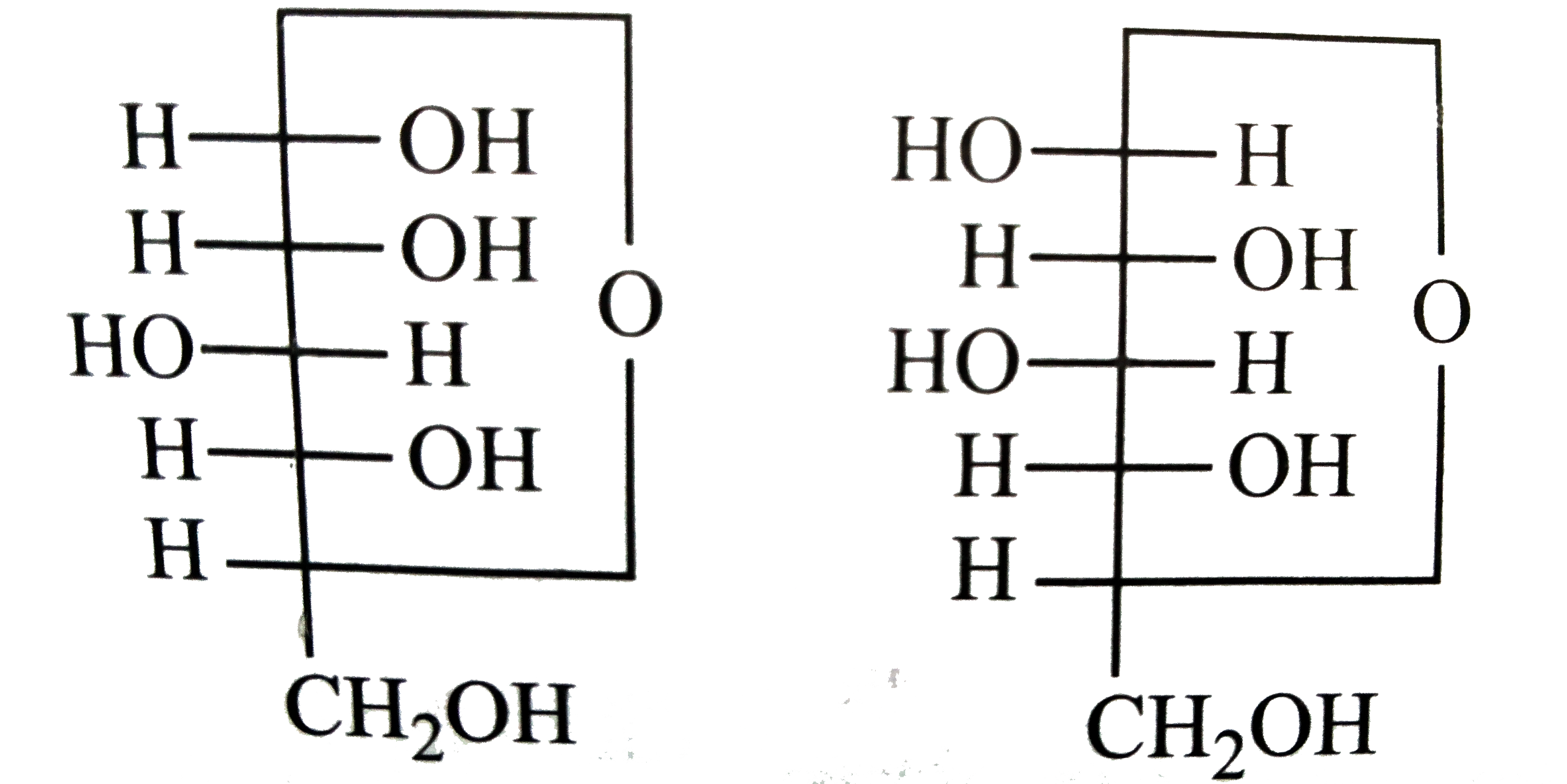 D
D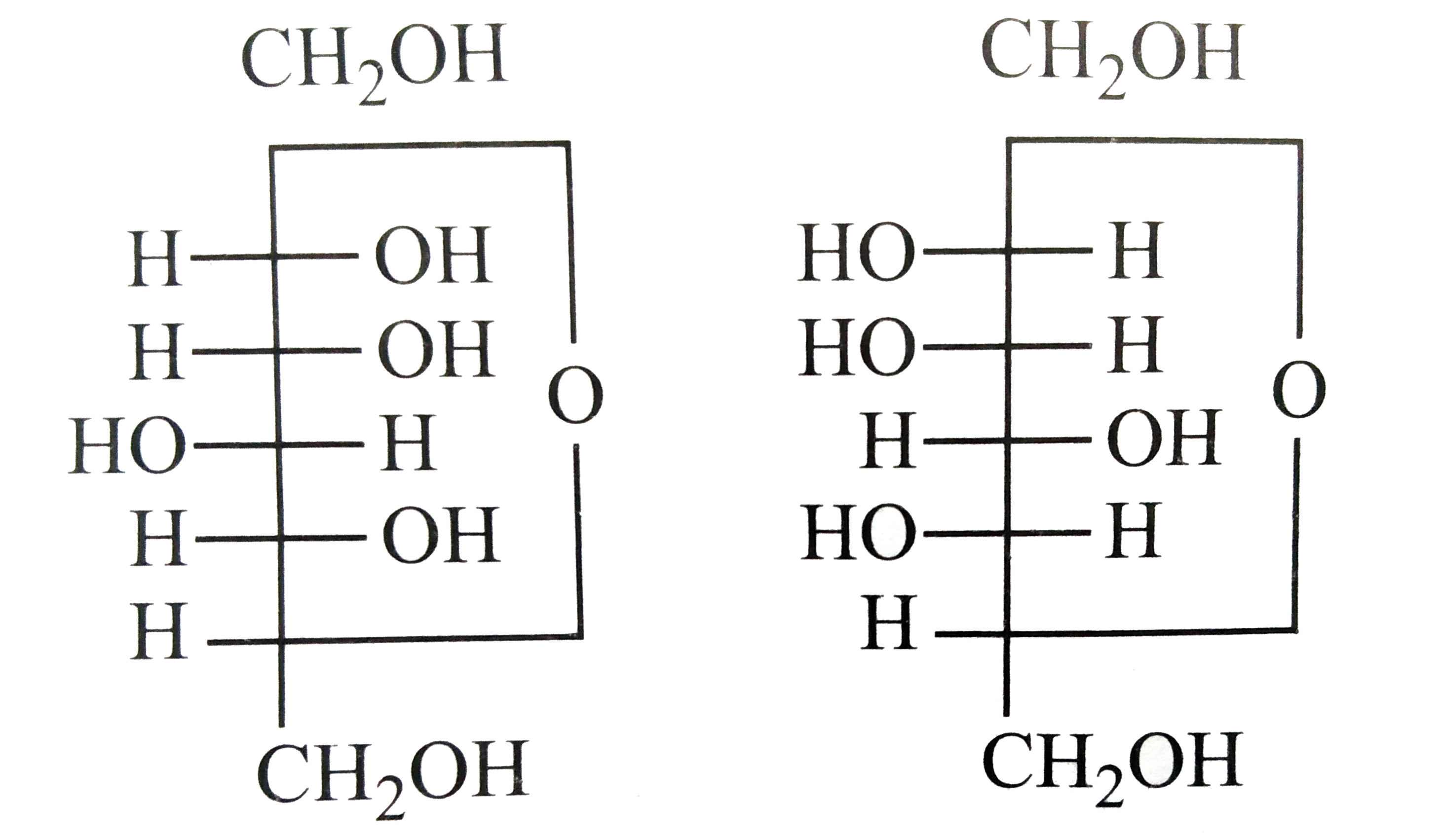
Which of the following pairs are geometrical isomers?
A1.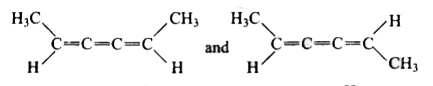 B2.
B2.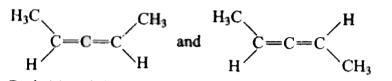 C3.Both of theseD4.None of the above
C3.Both of theseD4.None of the above
Which one of the following pair represents stereo isomerism :-
View SolutionWhich one of the following pairs represent stereoisomerism?
View SolutionWhich of the following pairs represents resonating structures ?
View SolutionWhich of the following pair(s) represent(s) the isoelectronic species ?
View SolutionWhich of the following pairs represents constitutional isomers ?
View SolutionWhich of the following pairs represent of the same physical quantities?
View Solution
