Number of chiral centers in chloramphenicol is :
(a) 1
(b) 2
(c) 3
(d) 4
To determine the number of chiral centers in chloramphenicol, we will follow these steps:
Step 1: Understand the Definition of Chiral Center
A chiral center (or chiral carbon) is a carbon atom that is attached to four different groups. This gives rise to non-superimposable mirror images, known as enantiomers.
Hint: Remember that for a carbon to be chiral, it must have four distinct substituents.
Step 2: Draw the Structure of Chloramphenicol
The chemical structure of chloramphenicol can be drawn or obtained from a chemical database. The structure consists of a benzene ring, a nitro group, and a hydroxyl group, among other components.
Hint: Visualizing the structure helps in identifying the substituents attached to each carbon atom.
Step 3: Identify the Carbons in the Structure
Look at the carbon atoms in the chloramphenicol structure. Identify each carbon and the groups attached to it.
Hint: Focus on each carbon atom and list the groups attached to it to see if they are different.
Step 4: Check Each Carbon for Chirality
For each carbon atom, check if it has four different groups attached:
- If a carbon has four different groups, it is a chiral center.
- If a carbon has two or more identical groups, it is not chiral.
Hint: Pay special attention to the carbons that are connected to functional groups, as they are more likely to be chiral.
Step 5: Count the Chiral Centers
After analyzing the structure, count the number of chiral centers identified. In chloramphenicol, you will find that there are two carbons that meet the criteria for chirality.
Hint: Double-check your count to ensure you haven’t missed any chiral centers.
Conclusion
The number of chiral centers in chloramphenicol is 2.
Final Answer: The correct option is 2.
A chiral center (or chiral carbon) is a carbon atom that is attached to four different groups. This gives rise to non-superimposable mirror images, known as enantiomers.
The chemical structure of chloramphenicol can be drawn or obtained from a chemical database. The structure consists of a benzene ring, a nitro group, and a hydroxyl group, among other components.
Look at the carbon atoms in the chloramphenicol structure. Identify each carbon and the groups attached to it.
For each carbon atom, check if it has four different groups attached:
- If a carbon has four different groups, it is a chiral center.
- If a carbon has two or more identical groups, it is not chiral.
After analyzing the structure, count the number of chiral centers identified. In chloramphenicol, you will find that there are two carbons that meet the criteria for chirality.
The number of chiral centers in chloramphenicol is 2.
Topper's Solved these Questions
Similar Questions
Knowledge Check
The numbers of chiral centers present in glucopyranose and fructofuranose are :
A4 and 3B5 and 4C4 in eachD5 in eachCH3−CH∣CH3−CH2−CH3Cl2−−−−−→hv
Number of chiral centers generated during monochlorination in the above reaction :A1B2C3D4
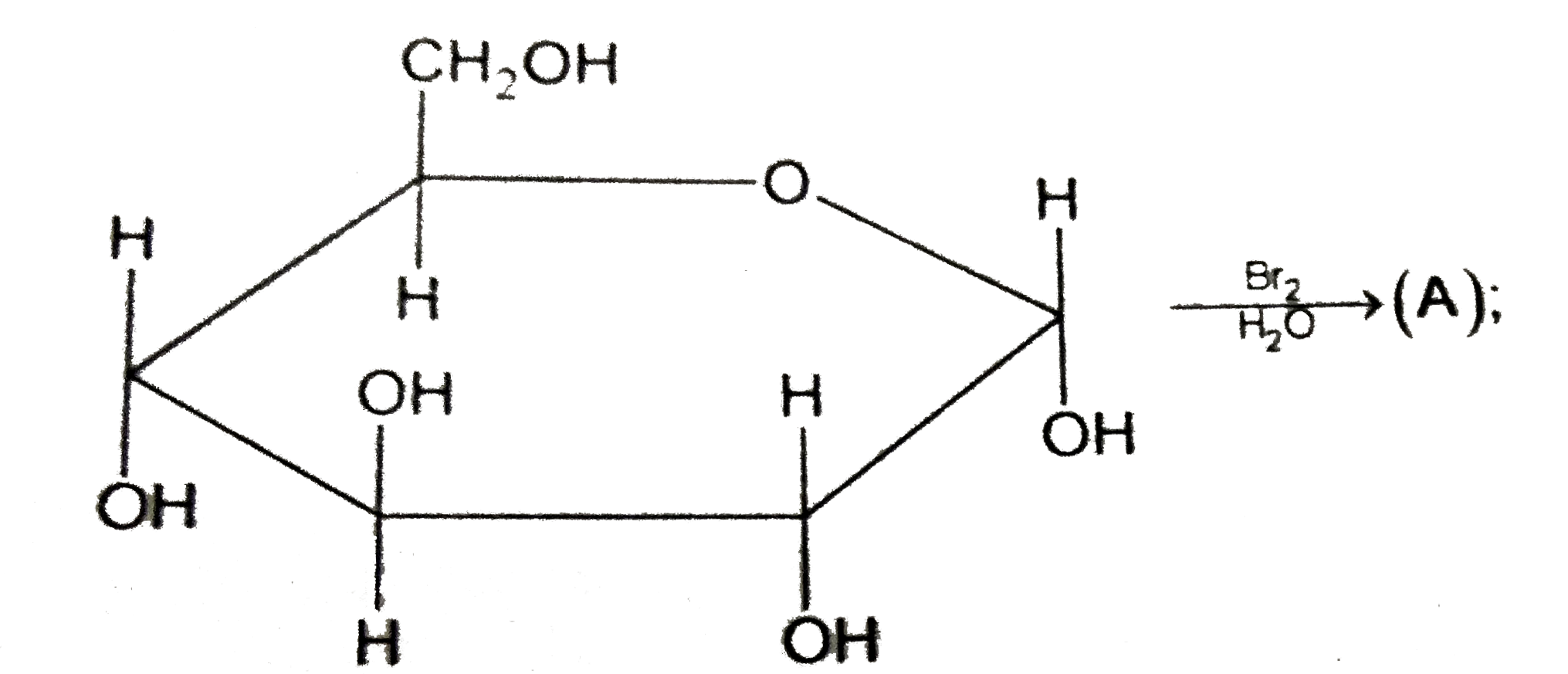 Br2−−→H2O (A), number of chiral centre in compound (A) is
Br2−−→H2O (A), number of chiral centre in compound (A) is
View SolutionThe number of chiral centres in the given compound is
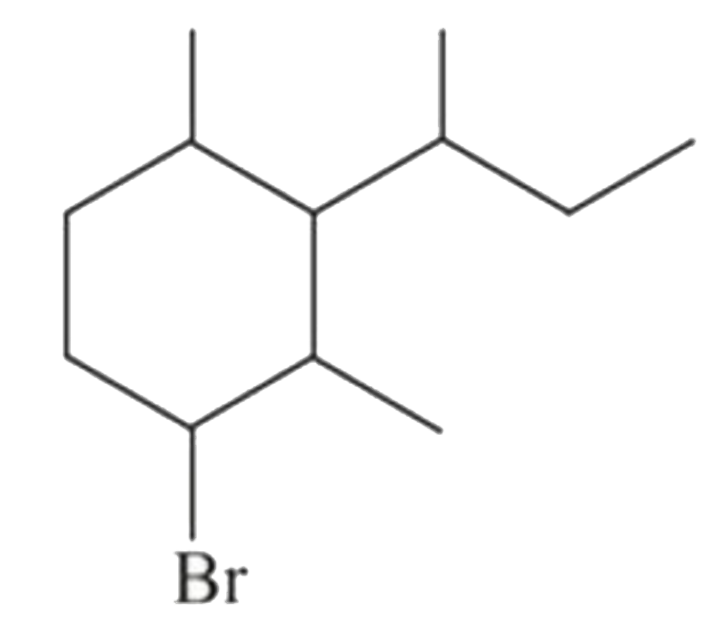
View SolutionThe number of chiral centres in D-(+)-glucose is
View SolutionNumber of chiral center of the given compound:-
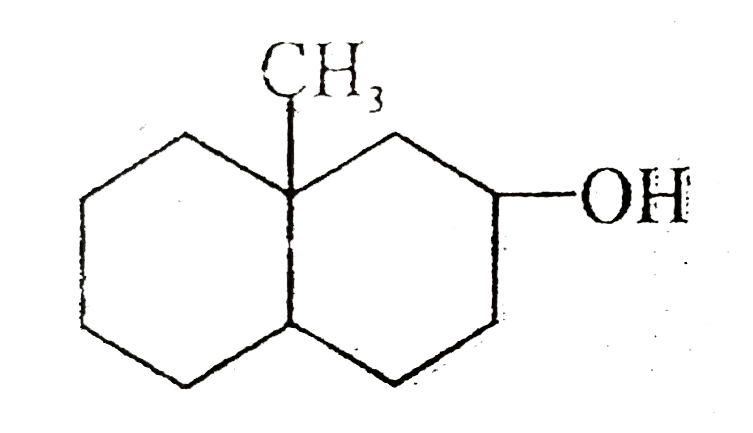
View Solutionwhat is chiral center?
View SolutionThe number of chiral carbon atoms in Aspartame is _________.
View Solution

