What are the functional groups present in the structure of maltose ?
One ketal and one hemiketal
One acetal and one ketal
One acetal and one hemiacetal
Two acetals
The correct Answer is:C
To determine the functional groups present in the structure of maltose, we need to analyze its molecular structure. Maltose is a disaccharide composed of two glucose units linked by an α(1→4) glycosidic bond. Here’s a step-by-step breakdown of the functional groups present in maltose:
Step 1: Identify the Structure of Maltose
Maltose consists of two glucose molecules. Each glucose unit has a specific structure that includes hydroxyl (–OH) groups and a cyclic structure.
Step 2: Recognize the Glycosidic Bond
The two glucose units in maltose are connected by a glycosidic bond. In maltose, this is specifically an α(1→4) glycosidic bond, which is formed between the first carbon of one glucose and the fourth carbon of the other glucose.
Step 3: Identify Functional Groups
1. Hydroxyl Groups (–OH): Each glucose unit contains multiple hydroxyl groups. In maltose, there are a total of 5 hydroxyl groups (one from each glucose and one from the hemiacetal).
2. Hemiacetal Group: The anomeric carbon (the carbon that was part of the carbonyl group in the open-chain form of glucose) of one glucose unit is involved in a hemiacetal formation. This is characterized by having one hydroxyl group and one ether bond (the glycosidic bond to the second glucose).
3. Ether Group: The glycosidic bond itself can be considered an ether linkage, where the oxygen atom connects the two glucose units.
Conclusion
The functional groups present in maltose are:
- Hydroxyl groups (–OH)
- Hemiacetal group
- Ether group (from the glycosidic bond)
Summary of Functional Groups in Maltose:
- Hydroxyl groups: 5 total
- Hemiacetal group: 1
- Ether group: 1 (from the glycosidic bond)
Maltose consists of two glucose molecules. Each glucose unit has a specific structure that includes hydroxyl (–OH) groups and a cyclic structure.
The two glucose units in maltose are connected by a glycosidic bond. In maltose, this is specifically an α(1→4) glycosidic bond, which is formed between the first carbon of one glucose and the fourth carbon of the other glucose.
1. Hydroxyl Groups (–OH): Each glucose unit contains multiple hydroxyl groups. In maltose, there are a total of 5 hydroxyl groups (one from each glucose and one from the hemiacetal).
2. Hemiacetal Group: The anomeric carbon (the carbon that was part of the carbonyl group in the open-chain form of glucose) of one glucose unit is involved in a hemiacetal formation. This is characterized by having one hydroxyl group and one ether bond (the glycosidic bond to the second glucose).
3. Ether Group: The glycosidic bond itself can be considered an ether linkage, where the oxygen atom connects the two glucose units.
The functional groups present in maltose are:
- Hydroxyl groups (–OH)
- Hemiacetal group
- Ether group (from the glycosidic bond)
- Hydroxyl groups: 5 total
- Hemiacetal group: 1
- Ether group: 1 (from the glycosidic bond)
Topper's Solved these Questions
Similar Questions
Thiol group is present in
View SolutionWhat are the functional groups in the C2H5OH
View SolutionWhat are the functional groups in the CH3CHO
View SolutionWhat is the functional group present in the CH3COOH
View SolutionName the functional group present in HCOOH.
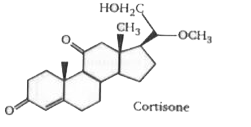
View SolutionThe functional groups present in Cortisone are :
View SolutionName the functional group present in CH3CHO .
View SolutionName the functional group present in C2H5Cl.
View SolutionName the functional group present in C2H5COOH
View SolutionThe functional group present in acetic acid is :
View Solution
