Explain Rutherford nuclear model of atom Solution in Gujrati
Video Solution
Text Solution
Rutherford and his students (Hans geiger and Ernest Marsden ) give α- particle scattering experiment
In this experiment they bombarded α particles on very thin gold foil. The experiment is shown below.
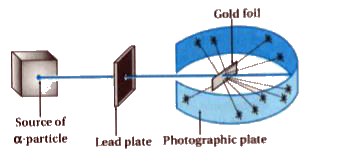
High energy α-particle from a radioactive source was direction at thin foil of gold metal
A fluoresecent zinc sulphide screen was kept around the thin gold foil then α particles struck this screen they produced fluorescence effect.
|
Similar Questions
Explain : Rutherford atomic model can not explain stability of an atom
View SolutionBased on his alpha -ray scattering experiment, Rutherford proposed the nuclear model of an atom. Write the important demerits of the Rutherford model.
View SolutionBased on alpha ray scattering experiment Rutherford proposed the nuclear model of atom.Give the main postulates of rutherford's nuclear model of an atom.
View SolutionRutherford atom model could not explain the _____ of the atom.
View SolutionAtomic Model|Result of Rutherford Nuclear Model
View SolutionBased on his α- ray scattering experiment, Rutherford proposed the nuclear model of an atom. Write the important demerits of the Rutherford model.
View SolutionRutherford's atomic model ....................... failed to explain.
View Solution

