Number of unpaired electron(s) present in an element having atom ic num ber Z = 24 is ......... Solution in Gujrati
A
6
B
4
C
0
D
5
Video Solution
Text Solution
The correct Answer is:A
Element (A=24) is chromium electron configuration is
Cr(Z=24)[Ar]183d54s1
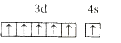
thus it has 6 unparied electrons
|
Similar Questions
Show the distribution of electrons in oxygen atom (atom ic num ber 8) using orbital diagram .
View SolutionShow the distribution of electrons in oxygen atom (atom ic num ber 8) using orbital diagram .
View SolutionThe number of unpaired electrons present in palladium (Z = 46) atom is
View SolutionThe number of unpaired electrons present in palladium (Z = 46) atom is
View SolutionAmong the elements from atomic number 1 to 36, the number of elements which have an unpaired electron in their S subshell is
View SolutionAmong the elements from atomic number 1 to 36, the number of elements which have an unpaired electron in their s-subshell is
View SolutionThe outer electronic configuration of Gd (Atomic number 64) is
View Solution

