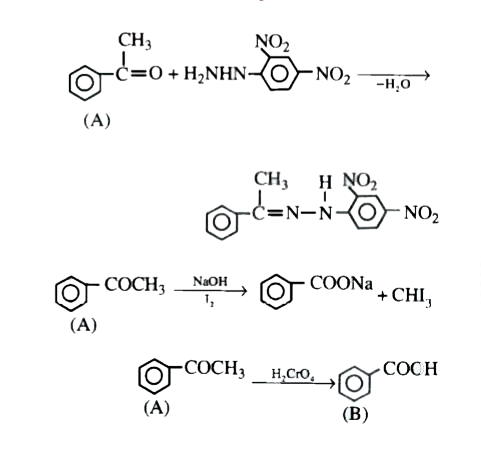
An organic compound (X) with molecular formula C8H8O forms an orange red precipitate with 2, 4 - DNP regent and resonds iodoform test. It neither decolourises Baeyer's reagent or bromine water and also it neither reduces Tollen's reagent or Fehling's reagent. Compound (X) gives a carboxylic acid (Y) with molecular formula C7H6O2 on drastic oxidation with chromic acid . Give the equations. Solution in Telugu
Topper's Solved these Questions
- ALDEHYDES AND KETONESBOOK - AAKASH SERIESCHAPTER - ALDEHYDES AND KETONESEXERCISE - Subjective exercise - 1 (Short answer Questions)
6 Videos
- ALDEHYDES AND KETONESBOOK - AAKASH SERIESCHAPTER - ALDEHYDES AND KETONESEXERCISE - Subjective exercise - 1 (Very short answer questions)
3 Videos
- ALDEHYDES AND KETONESBOOK - AAKASH SERIESCHAPTER - ALDEHYDES AND KETONESEXERCISE - PRACTICE SHEET-3
34 Videos
- ALCOHOLS, PHENOLS AND ETHERSBOOK - AAKASH SERIESCHAPTER - ALCOHOLS, PHENOLS AND ETHERSEXERCISE - ETHERS (OBJECTIVE EXERCISE-4)
50 Videos
- ALKYL AND ARYL HALIDESBOOK - AAKASH SERIESCHAPTER - ALKYL AND ARYL HALIDESEXERCISE - OBJECTIVE EXERCISE - 4 (ASSERTION (A) & REASON (R) TYPE QUESTIONS)
19 Videos
Similar Questions
What are aldehydes and ketones ? Give the structural differences .
View SolutionWrite the IUPAC names for the following
Cl∣CH2−CHO,Cl∣CH2−O∣∣C−CH3
View SolutionWhich of the following is likely to highest melting point
View Solution(A): Alcoholic fermentation involves conversion of sugar into ethanol by the action of yeast.
(R): Fermentation involves the liberation of CO2 gas.
View Solution(A) Towards SN2 reaction, order of reactivity is CH3Br>CH3CH2Br>(CH3)2CHBr>(CH3)3CBr.
(R) Greater the stability of carbocation, greater will be its ease of formation from alkyl halide and faster will be the rate of SN1 reaction.
View SolutionAn organic compound (A) with molecular formula C8H8O forms an orange red precipitate with 2,4 -DNP reagent and gives yellow precipitate on heating with iodine in the presence of sodium hydroxide . It neither reduces Tollen's reagent or Fehling's solution , nor does it decolourise bromine water or Baeyer's reagent. On drastic oxidation with chromic acid, it gives a carboxylic acid (B) having molecular formulae C7H6O2. Identify the compound (A) and (B) and explain the reactions involved .
View SolutionAn organic compound 'P' with jmolecular formula C_(8)H_(8)O forms an organic red ppt with 2,4-DNP gives yellow ppt. on heating with I_(2) in presence of NaOH . It neither reduces tollen's or fehling reagent nor does it decolourise Br_(2) water or baeyer's reagent. On drastic oxidation with chromic acid (H_(2)CrO_(4)) it gives an acid (Q) having molecular formula C_(7)H_(6)O_(2) . Q. Identify compound P:
View SolutionAn organic compound (A) with molecular formula C8H8O forms an orange-red precipitate with 2,4-DNP reagent and gives yellow precipitate on heating with iodine in the presence of sodium hydroxide. It neither reduces Tollens’ or Fehlings’ reagent, nor does it decolourise bromine water or Baeyer’s reagent. On drastic oxidation with chromic acid, it gives a carboxylic acid (B) having molecular formula C7H6O2. Identify the compounds (A) and (B) and explain the reactions involved.
View SolutionAn organic compound (A) with molecular formula C8H8O forms an orange red precipitate with 2,4 -DNP reagent and gives yellow precipitate on heating with iodine in the presence of sodium hydroxide . It neither reduces Tollen's reagent or Fehling's solution , nor does it decolourise bromine water or Baeyer's reagent. On drastic oxidation with chromic acid, it gives a carboxylic acid (B) having molecular formulae C7H6O2. Identify the compound (A) and (B) and explain the reactions involved .
View SolutionAn organic compound (A) with molecular formula C8H8O forms an orange-red precipitate with 2,4-DNP reagent and gives yellow precipitate on heating with iodine in the presence of sodium hydroxide. It neither reduces Tollens’ or Fehlings’ reagent, nor does it decolourise bromine water or Baeyer’s reagent. On drastic oxidation with chromic acid, it gives a carboxylic acid (B) having molecular formula C7H6O2. Identify the compounds (A) and (B) and explain the reactions involved.
View SolutionAn organic compound (A) with molecular formula C8H8O forms an orange red precipitate with 2,4 -DNP reagent and gives yellow precipitate on heating with iodine in the presence of sodium hydroxide . It neither reduces Tollen's reagent or Fehling's solution , nor does it decolourise bromine water or Baeyer's reagent. On drastic oxidation with chromic acid, it gives a carboxylic acid (B) having molecular formulae C7H6O2. Identify the compound (A) and (B) and explain the reactions involved .
View SolutionAn organic compound (X) with molecular formula C8H8O forms an orange red precipitate with 2, 4 - DNP regent and resonds iodoform test. It neither decolourises Baeyer's reagent or bromine water and also it neither reduces Tollen's reagent or Fehling's reagent. Compound (X) gives a carboxylic acid (Y) with molecular formula C7H6O2 on drastic oxidation with chromic acid . Give the equations.
View SolutionAn organic compound (A) with molecular formula C8H8O forms an orange precipitate with 2, 4 dinitrophenyl hydrazine and gives yellow precipitate on heating with iodine in presence of sodium hydroxide. It neither reduces Tollen's reagent nor Fehling solution and it also does not decolourise bromine water or Baeyer's reagent. On drastic oxidation with chromic acid it gives a carboxylic acid (B) having molecular formula C7H6O2. Identify the compound (A) and (B) and explain in detail the reactions involved
View SolutionAn organic compound Awithmo≤ca––rforμlaC_k H_8 O. forms an' orange red precipitate with 2,4 . DNP reagent and gives yellow precipitate an heating with iodine in the presence of sodium hydróxide. it neither reduces Tollen's or Fehling's reagent, nor does it decolonise bromine water or Balyer's reagent. On 'draśtic. oxidation with chromic acid, it gives a carboxylic acid (B) having molecular formula C_7 H_6 O_-2^primeIdentifythecompoundsAandB and explain the reactions involved..
View SolutionAn organic compound (A) with molecular formula C8H8O forms an orange red precipitate with 2, 4 dinitrophenyl hydrazine and gives yellow precipitate on heating with iodine in presence of sodium hydroxide. It neither reduces Tollen.s reagent nor Fehling solution and it also does not decolourise bromine water or Baeyer.s reagent.
On drastic oxidation with chromic acid, it gives a carboxylic acid (B) having molecular formula C7H6O2. Identify the compound (A) and (B) and explain in detail the reactions involved.
View Solution