Halogen atom present in benzene ring is ortho, para directing, but deactivating. Why?Solution in Telugu
Step by step video & image solution for Halogen atom present in benzene ring is ortho, para directing, but deactivating. Why? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- ALKYL AND ARYL HALIDESBOOK - AAKASH SERIESCHAPTER - ALKYL AND ARYL HALIDESEXERCISE - EXAMPLES
40 Videos
- ALKYL AND ARYL HALIDESBOOK - AAKASH SERIESCHAPTER - ALKYL AND ARYL HALIDESEXERCISE - SUBJECTIVE EXERCISE - 1 (SHORT ANSWER QUESTIONS)
6 Videos
- ALKYL AND ARYL HALIDESBOOK - AAKASH SERIESCHAPTER - ALKYL AND ARYL HALIDESEXERCISE - Exercise-1.1.4
8 Videos
- ALDEHYDES AND KETONESBOOK - AAKASH SERIESCHAPTER - ALDEHYDES AND KETONESEXERCISE - Exercise 3.2
41 Videos
- AMINES AND AZO COMPOUNDS BOOK - AAKASH SERIESCHAPTER - AMINES AND AZO COMPOUNDS EXERCISE - PRACTICE SHEET - 6 (Integer answer type Questions)
9 Videos
Similar Questions
Give the IUPAC names of the compounds. Classify them as alkyl, allylic, benzylic, vinylic and aryl halides and also as primary, secondary and tertiary halides.
(i)(CH3)3CCH2CH(Cl)CH3
(ii)CH3CH2CH=CHCH2Cl,
(iii)(CH3)2CHCH2CH=C(Cl)CH2CH3and
(iv)C6H5C(Cl)CH3)2
View SolutionGive IUPAC names of isobutyl chloride, secondary butyl chloride and tertiary butyl chloride.
View SolutionHow chlorobenzene is prepared from
(i) aniline and (ii) phenol ?
View SolutionGive the IUPAC names of the following compounds: (CH3)3CCH2CHO,CH3CH=CHCHO,CH3CH2COCH(C2H5)CH2CH2ClandCH3CH(OH)CH2COCH3
View SolutionHow many aromatic (six membered carbocyclic) isomers possible for MFC7H9N.
View SolutionIn the electrophilic substituion of benzene ring, the second substituent is directed by the group already present. Electron releasing groups (+I and +M) are ortho-para-directing and activating, whreas the electron withdrawing groups (-I and -M) are meta-directing and deactivating.
Halogens are placed under the category of +T (Tautomeric) groups because they have -Ibdyctuve abd +Mesomeric effect. These groups are deactivating but ortho-para-directing.
In the introduction of third group to the benzene ring, the product of minimum steric gindrance is formed.
Answer the follwing questions :
Ortho-Xylene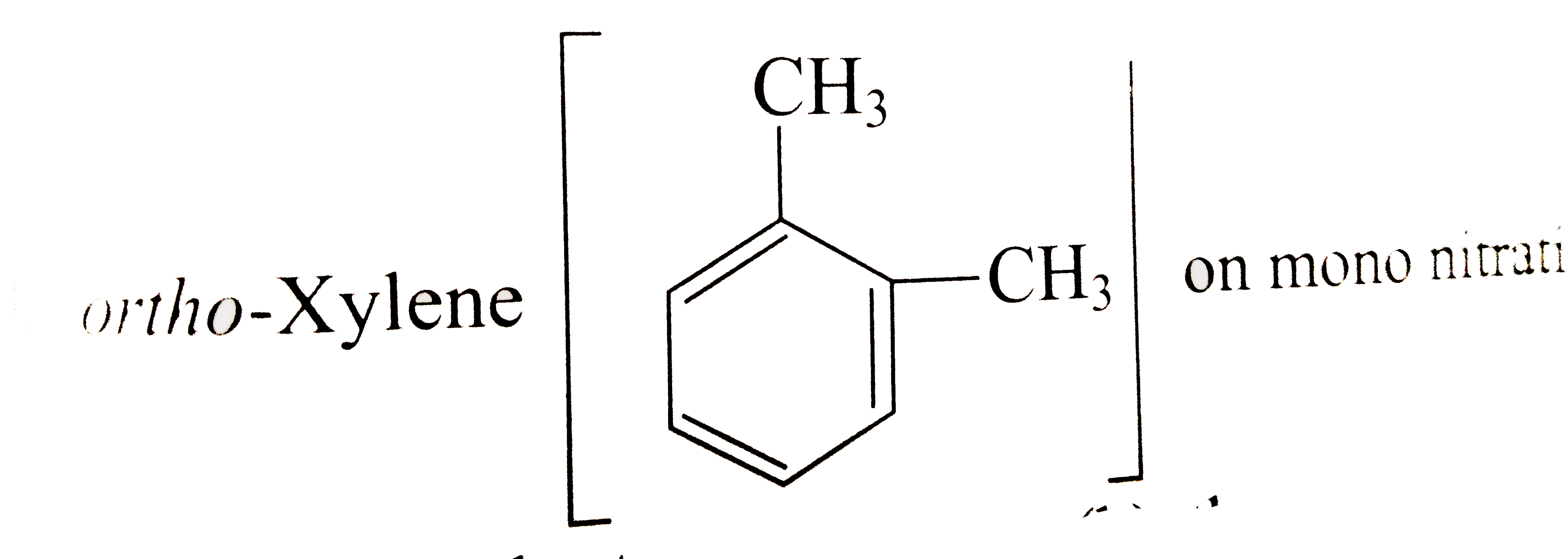 on mono nitration gives
on mono nitration gives
View SolutionIn the electrophilic substituion of benzene ring, the second substituent is directed by the group already present. Electron releasing groups (+I and +M) are ortho-para-directing and activating, whreas the electron withdrawing groups (-I and -M) are meta-directing and deactivating. Halogens are placed under the category of +T (Tautomeric) groups because they have -Ibdyctuve abd +Mesomeric effect. These groups are deactivating but ortho-para-directing. In the introduction of third group to the benzene ring, the product of minimum steric gindrance is formed. Which of the following is not an ortho, para-directing group ?
View SolutionIn the electrophilic substituion of benzene ring, the second substituent is directed by the group already present. Electron releasing groups (+I and +M) are ortho-para-directing and activating, whreas the electron withdrawing groups (-I and -M) are meta-directing and deactivating.
Halogens are placed under the category of +T (Tautomeric) groups because they have -Ibdyctuve abd +Mesomeric effect. These groups are deactivating but ortho-para-directing.
In the introduction of third group to the benzene ring, the product of minimum steric gindrance is formed.
A deactivating group in electrophilic substitution reaction :
View SolutionAssertion: In monohaloarenes, further electrophilic substitution occurs at ortho and para position
Reason: Halogen atom is a ring deactivator
View SolutionWhy is halogen atom when attached to benzene ring is ortho and para directing but somewhat deactivating in nature?
View SolutionAssertion: In monohaloarenes, further electrophilic substitution occurs at ortho and para positions. Reason: Halogen atom is a ring deactivator.
View SolutionAssertion: IN mono haloarenes, electrophilic substitution occurs at ortho and para positions.
Reason: Halogen atom is a ring deactivator
View SolutionAssertion (A) : In monohaloarenes, further electrophilic substitution occurs at ortho- and para- position. Reason (R) : Halogen atom is a ring deactivator.
View SolutionAssertion: In monohaloarenes, electrophilic substitution occurs at ortho and para positions.
Reason: Halogen atom is a ring deactivator.
View SolutionAssertion (A) : In monohaloarenes, further electrophilic substitution occurs at ortho and para positions.
Reason (R ). Halogen atom is a ring deactivator.
View Solution

