Figure show plot of PVT and P for 2gm of oxygen gas at two different temeratures.
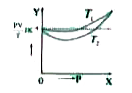
(i)The significance of dotted line
(ii)The relation between T1 and T2
(iii)The value of PVT where the curves meet on the Y-axis.Solution in Telugu
(i)For the dotted line ,PVT=constat.
It presents the behavior of an ideal gas.
(ii)The gas equation PV =RT is true only at high temperature In figure ,curve for T1 is closer to the dotted line than curve for T2
Hence the relatioon between T1 and T2 is T1>T2
(iii)PVT=μR.So the value of PVT where the curves meet on the Y-axis gives the value of μR.
In this caseμ=mM=232=116
∴PVT=μR=116×8.31=0.518J/K
Topper's Solved these Questions
- KINETIC THEORY OF GASESBook:AAKASH SERIESChapter:KINETIC THEORY OF GASESExercise:EXERCISE-IA (kinetic theory of an ideal a gas)
Explore 42 Videos
- KINETIC THEORY OF GASESBook:AAKASH SERIESChapter:KINETIC THEORY OF GASESExercise:EXERCISE-IA(Degree of Freedom)
Explore 35 Videos
- KINEMATICSBook:AAKASH SERIESChapter:KINEMATICSExercise:ADDITIONAL PRACTICE EXERCISE (PRACTICE SHEET (ADVANCED)) (Integer Type Questions)
Explore 12 Videos
- LAWS OF MOTIONBook:AAKASH SERIESChapter:LAWS OF MOTIONExercise:PRACTICE EXERCISE
Explore 108 Videos
Similar Questions
Pressure exerted by a gas is
View SolutionA cylinder rolls without slipping down an inclined plane, the number of degrees of freedom it has, is
View SolutionThree particles start from origin at the same time: one with velocity v1 along positive x - axis, the second along the positive y-axis with a velocity v2 and the third along the line y=x with such a speed that all the three always stay in a straight line, then the velocity of the third particle is √kV1V2(V1+V2).
View SolutionA force of 50 N acts on a body of mass 10 kg at rest. Its velocity after 5 seconds if the same force acts, is
View SolutionFig shows of PV/T versus P for 1.00×10−3kg of oxygen gas at two different temperatures.
(a) What does the dotted plot signify ?
(b) Which is true : T1<T2orT2<T1?
( c) What is the value of PV/T where the curves meet on the Y-axis ?
(d) If we obtained similar plot for 1.00×10−3kg of hydrogen, would we get the same value of PV/T at the point where the curves meet on the y-axis ? If not, what mass of hydrogen yield the same value of PV/T (for low pressure high temperature region of the plot) ? (Molecular mass of H=2.02u, of O=32.0u,R=8.31Jmol−1K−1
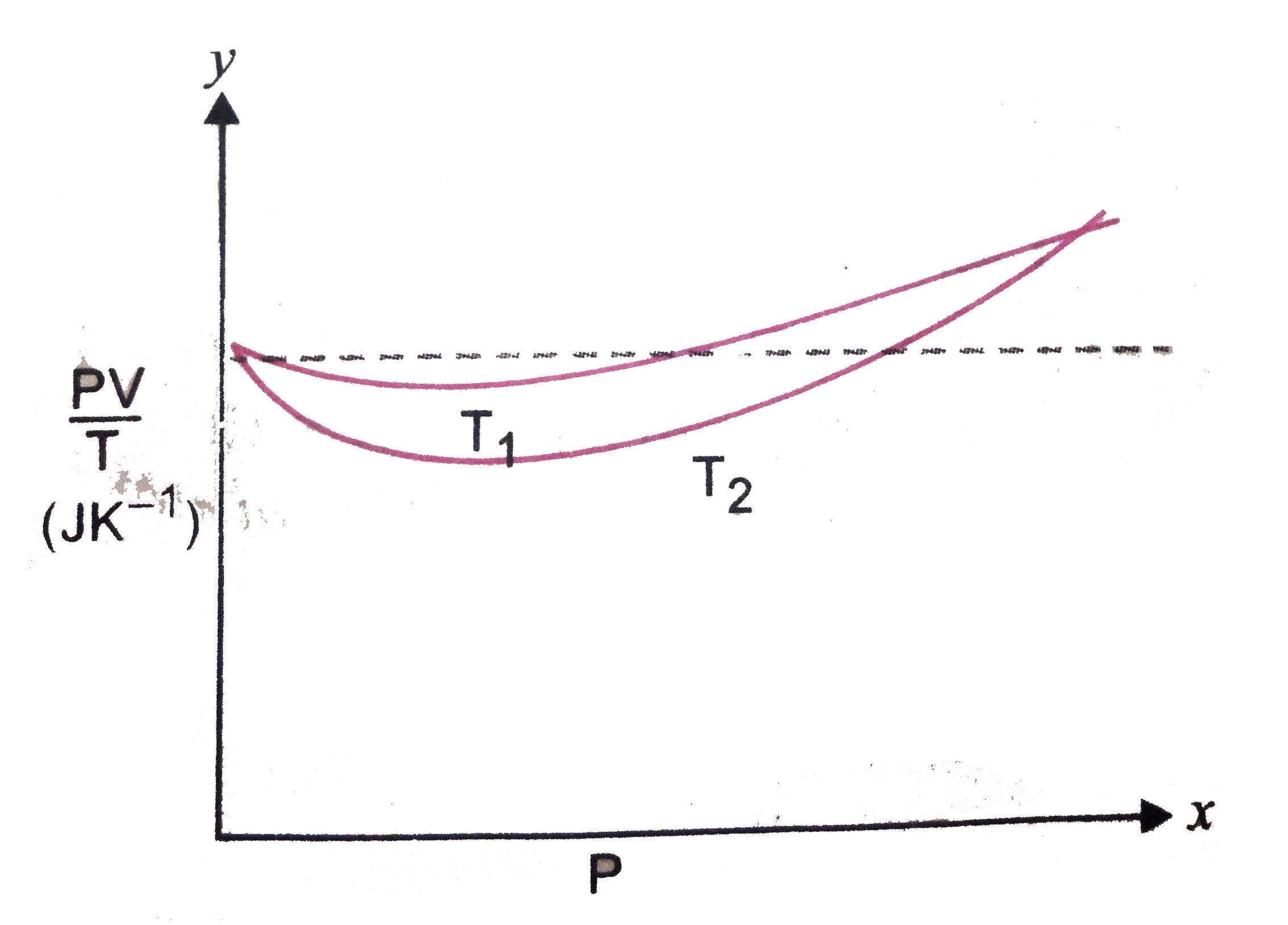
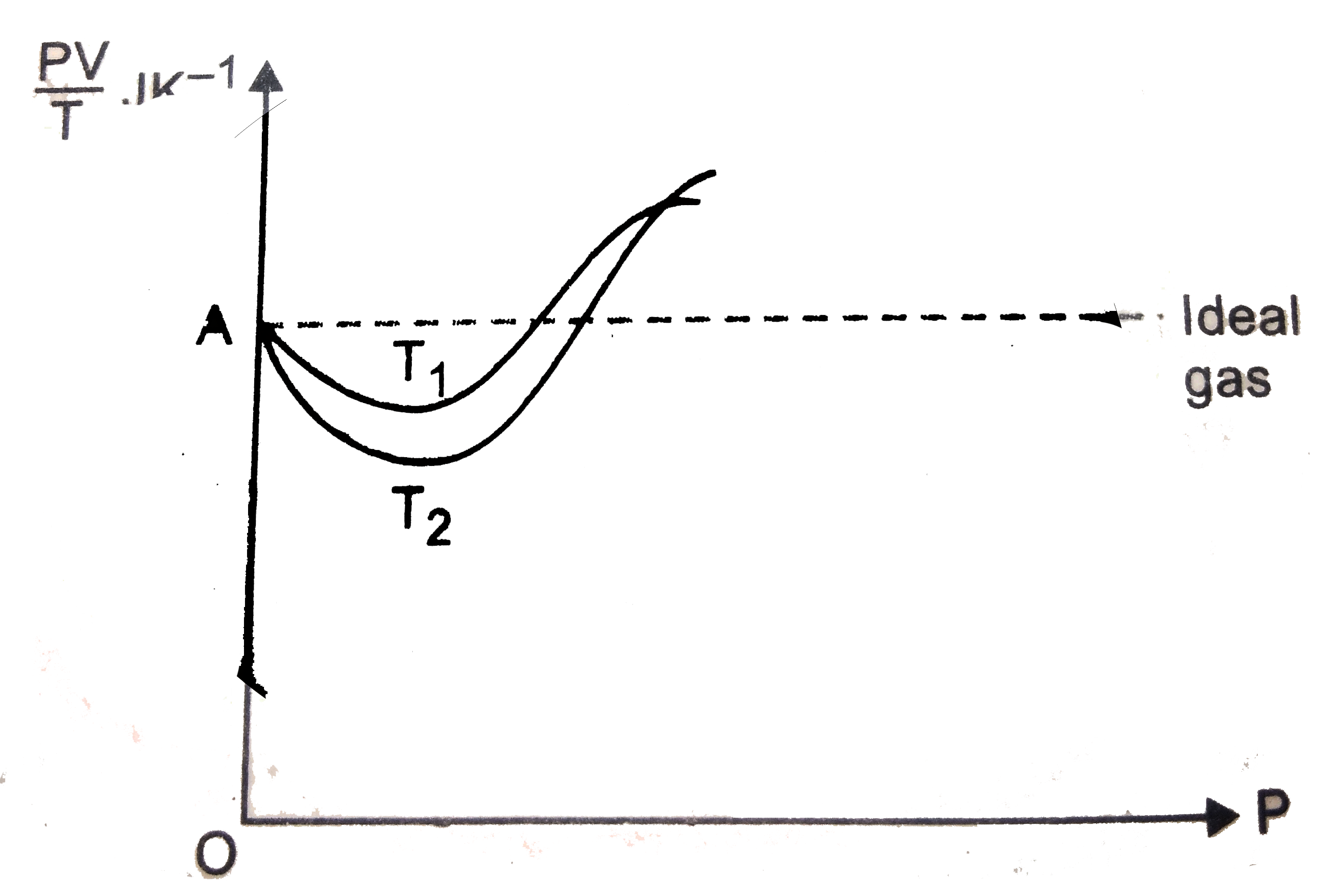
View SolutionGiven is the graph between PVT and P for 1 gm of oxygen gas at two different temperatures T1andT2 Fig. Given, density of oxygen =1.427kgm−3. The value of (PV)/(T) at the point A and the relation between T1andT2 are respectively :
View SolutionFigure shows plot of PV//T versus P"for" 1.00 xx 10^(-3) kg of oxygen gas at two different temperatures. (i) What does the dotted plot signify? (ii) Which is true . T_(1) gt T_(2) "or" T_(1) lt T_(2) ? (iii) What is the value of PV//T where the curves meet on the y-axis?
View Solutionचित्र में ऑक्सीजन के द्रव्यमान के लिए PV/T एवं P में , दो अलग - अलग तापों पर ग्राफ दर्शाये गए है ।
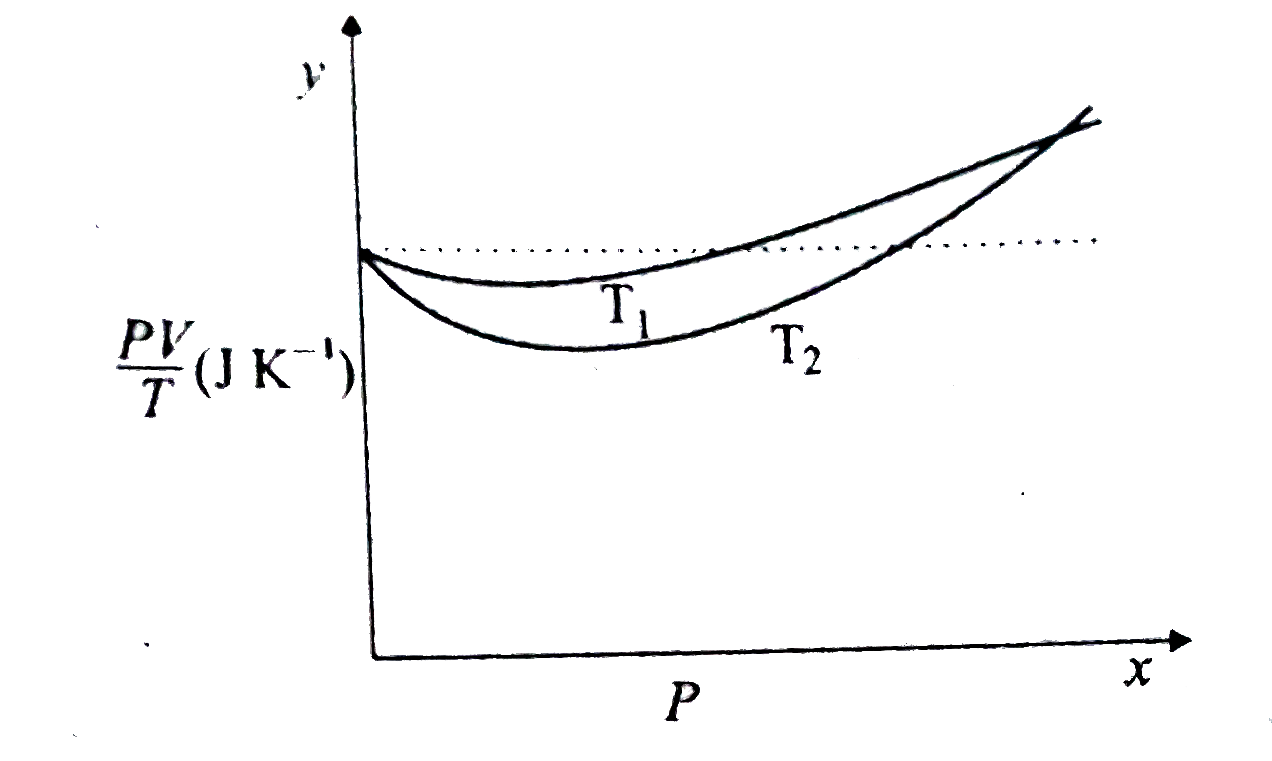
(a ) बिंदुकित रेखा क्या दर्शाती है ?
(b ) क्या सत्य है: T1>T2 अथवा T1<T2?
(c ) y - अक्ष पर जहाँ वक्र मिलते है वहाँ PV/T का मान क्या है ?
( d ) यदि हम ऐसे ही ग्राफ 1.00×10−3kg हाइड्रोजन के लिए बनाएँ तो भी क्या उस बिंदु पर जहाँ वक्र y - अक्ष से मिलते है PV/T का मान यही होगा ? यदि नहीं तो हाइड्रोजन के कितने द्रव्यमान के लिए PV/T का मान कम दाब और उच्च ताप के क्षेत्र के लिए वही होगा? (H2 का अणु द्रव्यमान =2.02u,O2 का अणु द्रव्यमान =32.0u,R=8.31Jmol−1K−1)
View SolutionThe figure below shows the plot of (PV)/(nT) versus P for oxygen gas at two different temperatures (##ARH_EGN_PRG_PHY_C17_E01_014_Q01.png" width="80%"> Read the following statements concerning the above curves. (i) The dotted line corresponds to the ideal gas behaviour (ii) T_(1)gtT_(2) The value of (pV)/(nT) at the point where the curves meet on the y-axis is the same for all gases Which of the above statements is true ?
View SolutionFigure Show plot of PV/T versus P for 1.00×10−3 kg of oxygen gas at two different temperatures.
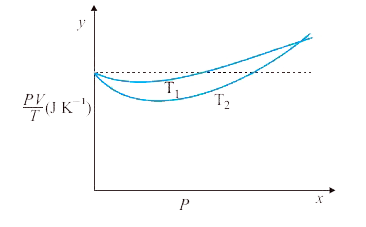
(a) What does the dotted plot signify?
(b) Which is true: T1>T2 or T1<T2?
(c) What is the value of PV/T where the curves meet on the y-axis?
(d) If we obtained similar plots for 1.00×10−3 kg of hydrogen, would we get the same value of PV/T at the point where the curves meet on the y-axis? If not, what mass of hydrogen yields the same value of PV/T (for low pressure high temperature region of the plot) ? (Molecular mass of H2=2.02u,ofO2=32.0u,R=8.31Jmol−1K−1.)
View SolutionFigure Show plot of PV/T versus P for 1.00×10−3 kg of oxygen gas at two different temperatures.
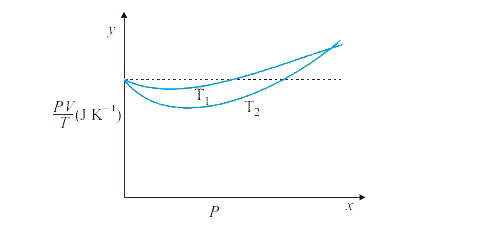
(a) What does the dotted plot signify?
(b) Which is true: T1>T2 or T1<T2?
(c) What is the value of PV/T where the curves meet on the y-axis?
(d) If we obtained similar plots for 1.00×10−3 kg of hydrogen, would we get the same value of PV/T at the point where the curves meet on the y-axis? If not, what mass of hydrogen yields the same value of PV/T (for low pressure high temperature region of the plot) ? (Molecular mass of H_(2) = 2.02 u, of O_(2) = 32.0 u, R= 8.31Jmol^(-1)K^(-1).)
View SolutionFigure Show plot of PV/T versus P for 1.00×10−3 kg of oxygen gas at two different temperatures.
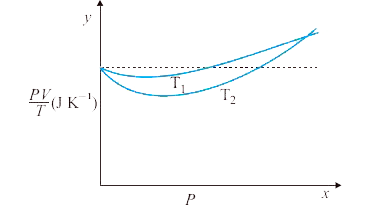
(a) What does the dotted plot signify?
(b) Which is true: T1>T2 or T1<T2?
(c) What is the value of PV/T where the curves meet on the y-axis?
(d) If we obtained similar plots for 1.00×10−3 kg of hydrogen, would we get the same value of PV/T at the point where the curves meet on the y-axis? If not, what mass of hydrogen yields the same value of PV/T (for low pressure high temperature region of the plot) ? (Molecular mass of H_(2) = 2.02 u, of O_(2) = 32.0 u, R= 8.31Jmol^(-1)K^(-1).)
View SolutionFigure Show plot of PV/T versus P for 1.00×10−3 kg of oxygen gas at two different temperatures.
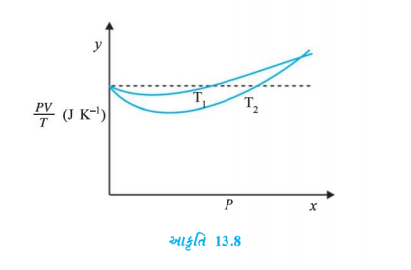
(a) What does the dotted plot signify?
(b) Which is true: T1>T2 or T1<T2?
(c) What is the value of PV/T where the curves meet on the y-axis?
(d) If we obtained similar plots for 1.00×10−3 kg of hydrogen, would we get the same value of PV/T at the point where the curves meet on the y-axis? If not, what mass of hydrogen yields the same value of PV/T (for low pressure high temperature region of the plot) ? (Molecular mass of H_(2) = 2.02 u, of O_(2) = 32.0 u, R= 8.31Jmol^(-1)K^(-1).)
View SolutionThe figure below shows the plot of versus P for oxygen gas at two different temperatures Read the following statements concerning the curves given below i)The dotted line corresponds to the 'ideal' gas behaviour ii) T_(1)gtT_(2) iii)The value of (PV)/(nT) at the point ,where the curves meet on the y-axis is the same for all gases which of the above statement is true?
View SolutionGiven is the graph between PV/T and P for 1 gm of oxygen gas at two different temperature T_(1) and T_(2) (Given:Density of oxygen= 1.429 kg//m^(3) ).The value of PV/T at the point A and the relation between T_(1) and T_(2) and respectively
View Solution
