A condenser of 21μF is charged to 100V and then discharged through a wire of mass 0.25 gm and specific heat 0.1 cal gm−1∘C−1. The rise in temperature of the wire is
0.5∘C
1∘C
1.5∘C
2∘C
The correct Answer is:B
Topper's Solved these Questions
- CAPACITORS Book:AAKASH SERIESChapter:CAPACITORS Exercise:LECTURE SHEET (EXERCISE - II) (LEVEL - I (ADVANCED)) (More than one correct answer Type Questions )
Explore 2 Videos
- CAPACITORS Book:AAKASH SERIESChapter:CAPACITORS Exercise:LECTURE SHEET (EXERCISE - II) (LEVEL - I (ADVANCED)) (Linked ComprehensionType Questions )
Explore 7 Videos
- CAPACITORS Book:AAKASH SERIESChapter:CAPACITORS Exercise:LECTURE SHEET (EXERCISE - II) (LEVEL - I (MAIN)) (Straight Objective Type Questions )
Explore 6 Videos
- ATOMSBook:AAKASH SERIESChapter:ATOMSExercise:PRACTICE EXERCISE
Explore 21 Videos
- COMMUNICATION SYSTEM Book:AAKASH SERIESChapter:COMMUNICATION SYSTEM Exercise:LECTURE SHEET (LEVEL - I) (MAIN) (EXERCISE - IV) (Straight Objective & More than one Correct Answer Type Questions)
Explore 20 Videos
Similar Questions
Identify the correct statements.
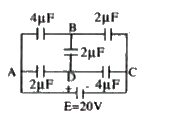
View SolutionThe given circuit shows an arrangement of four capacitors A potential difference 30V is applied across the combination. It is observed that potentials at points .A. and .B. differ by 5V. Also if a conducting wire is connected between .A. and .B. electrons will flow from A to B. Of course, we have not connected any wire actually between A and B, we have described only an .if. situation.
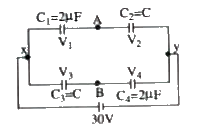
Potential difference across C4 is
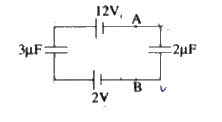
View SolutionThe P.D in volts between the points A and B is
View SolutionThe ground state energy of hydrogen atom is - 13.6eV. The kinetic energy of the electron in this state is:
View SolutionThe highest frequency of radiowaves which when sent at some angle towards the ionosphere , gets reflected from that and returns to the earth is called
View SolutionA copper ball of mass 100 gm is at a temperature T. It is dropped in a copper calorimeter of mass 100 gm, filled with 170 gm of water at room temperature. Subsequently, the temperature of the system is found to be 75^(@)C . T is given by : (Given : room temperature = 30^(@)C , specific heat of copper =0.1 cal//gm^(@)C )
View SolutionThe quantity of heat which can rise the temperature of xgm of a substance through t∘1C can rise the temperature of ygm of water through t∘2C is same. The ratio of specific heats of the substances is
View SolutionA copper block of mass 500gm and specific heat 0.1cal/gm∘C heated from 30∘C to 290∘C, the thermal capacity of the block is
View Solution10 gm of ice at – 20^(@)C is added to 10 gm of water at 50^(@)C . Specific heat of water = 1 cal//g–.^(@)C , specific heat of ice = 0.5 cal//gm-.^(@)C . Latent heat of ice = 80 cal/gm. Then resulting temperature is -
View SolutionA condenser of 21 mu F is charged to 100V and then discharged through a wire of mass 0.25gm and specific heat 0.1(cal)/(gc^(0)) . The rise in temperature of the wire is ......... C^0
View SolutionA copper ball of mass 100 gm is at a temperature T. It is dropped in a copper calorimeter of mass 100 gm, filled with 170 gm of water at room temperature. Subsequently, the temperature of the system is found to be 75^(@)C . T is given by : (Given : room temperature = 30^(@)C , specific heat of copper =0.1 cal//gm^(@)C )
View SolutionThe quantity of heat which can rise the temperature of x gm of a substance through t_(1)""^(@)C can rise the temperature of y gm of water through t_(2)""^(@)C is same. The ratio of specific heats of the substances is
View Solution10 gm of ice at −10∘C is mixed with 100 gm of water at 50∘C contained in a calorimeter weighing 50 gm. (Specific heat of water = 1cal.gm−1.∘C−1, Latent heat of ice = 80cal/gm, specific heat of ice = 0.5cal/gm/∘C and specific heat of copper = 0.09cal/gm/∘C). The final temperature reached by the mixtures is
View SolutionA copper block of mass 500 gm and specific heat 0.1cal/gm∘C is heated from 30∘C to 290∘C , the thermal capacity of the block is
View SolutionThe specific heat of a substance is 0.09 "cal/gm"^@C . If the temperature is measured on Fahrenheit scale the value of its specific heat is "cal/gm/"^@F is
View Solution10 gm of ice at - 10∘C is mixed with 100 gm of water at 50∘C contained in a calorimeter weighing 50gm. (Specific heat of water = 1cal gm−1.∘C−1 Latent heat of ice = 80 cal/gm, specific heat of ice = 0.5cal/gm0C and specific heat of copper =0.09cal/gm/∘C ). The final temperature reached by the mixtures is
View Solution

