Explain the formation of covalent bonds iu oxygen molecule (O2).
Text Solution
The atomic number of oxygen is 8, so its electronic configuration is KL26
Thus, oxygen has six electrons in its L shell.
Therefore, it needs two more electrons to fill the L shell.
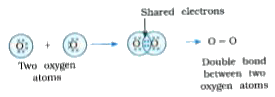
Hence, two atoms of oxygen share two electrons of L shell to form a molecule of oxygen (O2).
As a result, each atom of oxygen attains the electronic configuration of their nearest noble gas neon (Ne), which has eight electrons in their L shell.
These four valence electrons are depicted by dots or crosses is shown as follows :
|
Topper's Solved these Questions
- CARBON AND ITS COMPOUNDSBook:KUMAR PRAKASHANChapter:CARBON AND ITS COMPOUNDSExercise:ACTIVITY
Explore 22 Videos
- CARBON AND ITS COMPOUNDSBook:KUMAR PRAKASHANChapter:CARBON AND ITS COMPOUNDSExercise:TEXTUAL EXERCISE
Explore 14 Videos
- ACID , BASES AND SALTBook:KUMAR PRAKASHANChapter:ACID , BASES AND SALTExercise:Activity
Explore 47 Videos
- CHEMICAL REACTIONS AND EQUATIONS Book:KUMAR PRAKASHANChapter:CHEMICAL REACTIONS AND EQUATIONS Exercise:PRACTICAL SKILL BASED QUESTIONS WITH ANSWERS
Explore 5 Videos
Similar Questions
Knowledge Check
Multiple covalent bonds exist in a molecule of
AF2BN2CCH4DH2Multiple covalent bonds exist in a molecule of
AF2BN2CCH4DH2
Describe the formation of oxygen molecule (O2).
View SolutionMultiple covalent bonds exist in a molecule of
View SolutionExplain the formation of a covalent bond. State two factors which influence the formation of a covalent bond.
View SolutionExplain formation of covalent bond by the electron structure of hydrogen, oxygen and nitrogen molecules.
View SolutionExplain the formation of H2 molecule on the basis of valance bond theory.
View SolutionExplain the formation of H2 molecule on the basis of valance bond theory.
View Solution
