A molecule of NH3 (ammonia) has …
A
only single bonds.
B
only double bonds.
C
only triple bonds.
D
two double bonds and one single bond.
Text Solution
The correct Answer is:A
|
Similar Questions
Knowledge Check
A molecule of ammonia (NH3) has ( अमोनिया अणु है )
AOnly single bondsBOnly double bondsCOnly triple bondsDtwo double bonds and one single bondA molecule of ammonia (NH3) has ( अमोनिया अणु है )
Aonly single bondsBonly double bondsConly triple bondsDtwo double bonds and one single bond.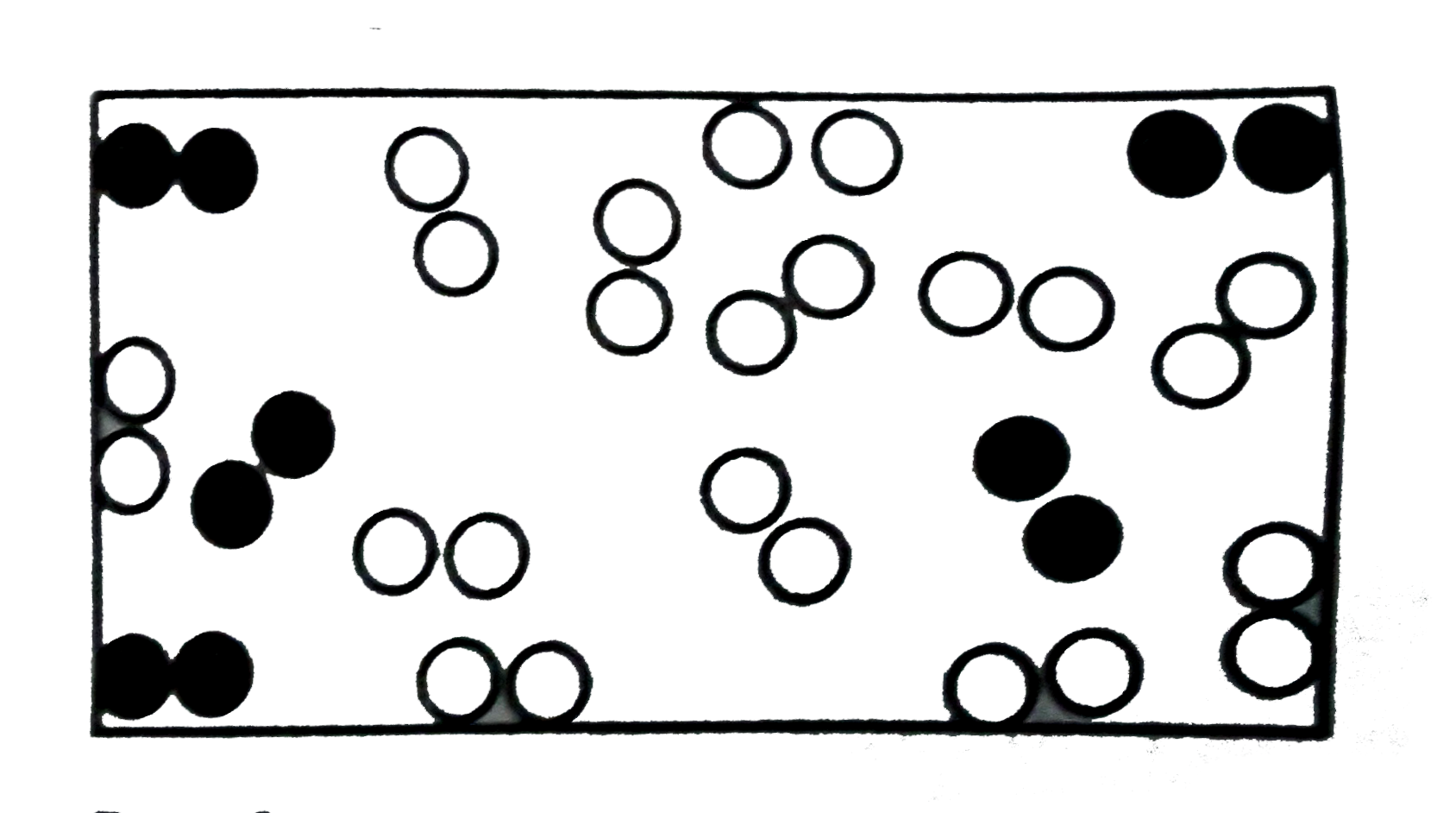
In the above diagram, the paired open spheres represent H2 molecules and the paired solid spheres represent N2 molecules. When the molecules in the box react to form the maximum possible amount of ammonia (NH3 molecules, what is the limiting reactant and how many molecules of NH3 can be formed?AN2 is limiting. 5 molecules of NH3 can be formed.BN2 is limiting. 10 molecules of NH3 can be formed.CH2 is limiting. 8 molecules of NH3 can be formed.DH2 is limiting, 12 molecules of NH3 can be formed.
A gas jar contains 7.2×1020 molecules of NH3 gas. Find :
volume in cm3 of ammonia gas at S. T.P.
[N = 14, H = 1]
View SolutionConsidering the van der Waals equation of state (P+a/V2)(V−b)=RT for ammonia (NH3) and nitrogne (N2) the value of a for NH3 is larger than that of N2
Ammonia has a lower molecular weight than nitrogen .
View SolutionCalculate the molecular mass of NH3 molecule.
View SolutionDuring N2 fixation, reduction of one molecule of nitrogen into 2 molecules of NH3 consumes _____molecules of ATP.
View SolutionReduction of one molecule of nitrogen into 2 molecules of NH3 consumes
View Solution
