One litre of buffer solution contains 0.1 mole of acetic acid add 1 mole of sodium acetate. Find its pH if pKa of CH3COOH is 4.8.Solution in Telugu
pH=pKa+log[salt][acid]
[ Salt ] =[Acid] =1
∴pH=4.8=pKa
mole of NaOH added to the solution = 4/40 = 0.1
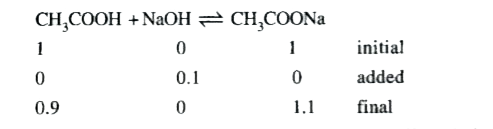
After adding 0.1 mole of caustic soda to the buffer solution
pH=4.8+log1.10.9=4.8+log1.222=4.886
Similar Questions
A buffer solution contains 0.1 M each of acetic acid and sodium acetate. The pH of the buffer solution is (pK_(a)(CH_(3)COOH)=4.75) :
View SolutionA buffer solution contains 0.1 mole of sodium acetate dissolved in 1000cm3 of 0.1 M acetic acid. To the above buffer solution, 0.1 mole of sodium acetate is further added and dissolved. The pH of the resulting buffer is
View SolutionA buffer solution contains 0.1 mole of sodium acetate dissolved in 1000cm3 of 0.1 M acetic acid. To the above buffer solution, 0.1 mole of sodium acetate is further added and dissolved. The pH of the resulting buffer is
View SolutionA buffer solution contains 0.1 mole of sodium acetate dissolved in 1000cm3 of 0.1 M acetic acid. To the above buffer solution, 0.1 mole of sodium acetate is further added and dissolved. The pH of the resulting buffer is
View SolutionA buffer solution contains 0.1 mole of sodium acetate dissolved in 1000cm3 of 0.1 M acetic acid. To the above buffer solution, 0.1 mole of sodium acetate is further added and dissolved. The pH of the resulting buffer is
View SolutionA buffer solution contains 0.1 mole of sodium acetate dissolved in 1000cm3 of 0.1 M acetic acid. To the above buffer solution, 0.1 mole of sodium acetate is further added and dissolved. The pH of the resulting buffer is
View SolutionA buffer solution contains 0.1 mole of sodium acetate dissolved in 1000cm3 of 0.1 M acetic acid. To the above buffer solution, 0.1 mole of sodium acetate is further added and dissolved. The pH of the resulting buffer is
View Solution50 ml o 0.1 M sodium acetate, 25 ml of 0.2 M acetic acid were added together to form the buffer solution. pKa of CH3COOH is 4.8. Find the pH of the solution.
View Solution

