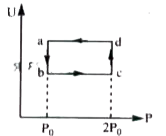
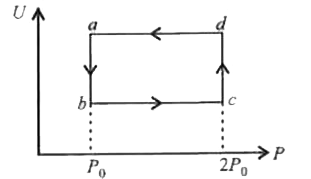
Figure shows variation of internal energy (U) with the pressure (P) of 2.0 mole gas in cyclic process abcda. The temperature of gas at c and d are 300 K and 500 K. Heat absorbed in the process is 400 R in X. Find the value of X.
 Solution in Telugu
Solution in Telugu
3
1
-2
2
The correct Answer is:2
Step by step video & image solution for Figure shows variation of internal energy (U) with the pressure (P) of 2.0 mole gas in cyclic process abcda. The temperature of gas at c and d are 300 K and 500 K. Heat absorbed in the process is 400 R in X. Find the value of X. by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- THERMODYNAMICSBook:AAKASH SERIESChapter:THERMODYNAMICSExercise:LECTURE SHEET (EXERCISE - II (STRAIGHT OBJECTIVE TYPE QUESTIONS))
Explore 10 Videos
- THERMODYNAMICSBook:AAKASH SERIESChapter:THERMODYNAMICSExercise:LECTURE SHEET (EXERCISE - II (MORE THAN ONE CORRECT ANSWER TYPE QUESTIONS))
Explore 5 Videos
- THERMODYNAMICSBook:AAKASH SERIESChapter:THERMODYNAMICSExercise:LECTURE SHEET (EXERCISE - I (MATRIX MATCHING TYPE QUESTIONS))
Explore 1 Video
- THERMAL PROPERTIES OF MATTERBook:AAKASH SERIESChapter:THERMAL PROPERTIES OF MATTERExercise:ADDITIONAL PRACTICE EXERCISE (LEVEL - II) PRACTICE SHEET (ADVANCED) Integer/Subjective Type Questions
Explore 2 Videos
- THERMOMETRY Book:AAKASH SERIESChapter:THERMOMETRY Exercise:Numerical Exercise (LEVEL- 1)
Explore 11 Videos
Similar Questions
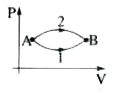
The figure shows two paths for the change of state of a gas from A to B. The ratio of molar heat capacities in path 1 and path 2 is:
View SolutionOne mole of an ideal gas undergoes a process such that P∝1√T . The molar heat capacity of this process is 4R, where R is universal gas constant. Then,
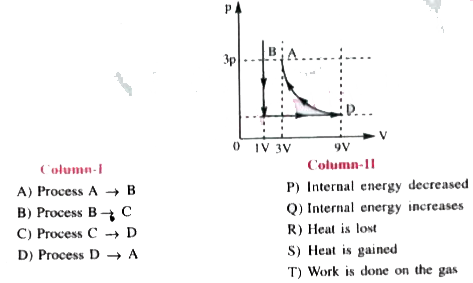
View SolutionOne mole of a monoatomic ideal gas is taken through a cycle ABCDA as shown on the P, V diagram, Column-II give the characteristics involved in the cycle. Match them with each of the process given in Column-I.
View SolutionA constant pressure gas thermometer has a volume of 200cc, 273.2cc and 525 .1cc at 0°C, 100°C and boiling point of sulphur respectively. The resistance possessed by a platinum wire at the same temperatures as above are 2.000Ω,2.778Ωand5.280Ω respectively. Estimate the boiling point of sulphur on the basis of the two sets of observations.
View SolutionIf a temperature scale defines the freezing point of water as 100∘C and the boiling as 0∘ what temperature on the scale corresponds to 25∘ C ?
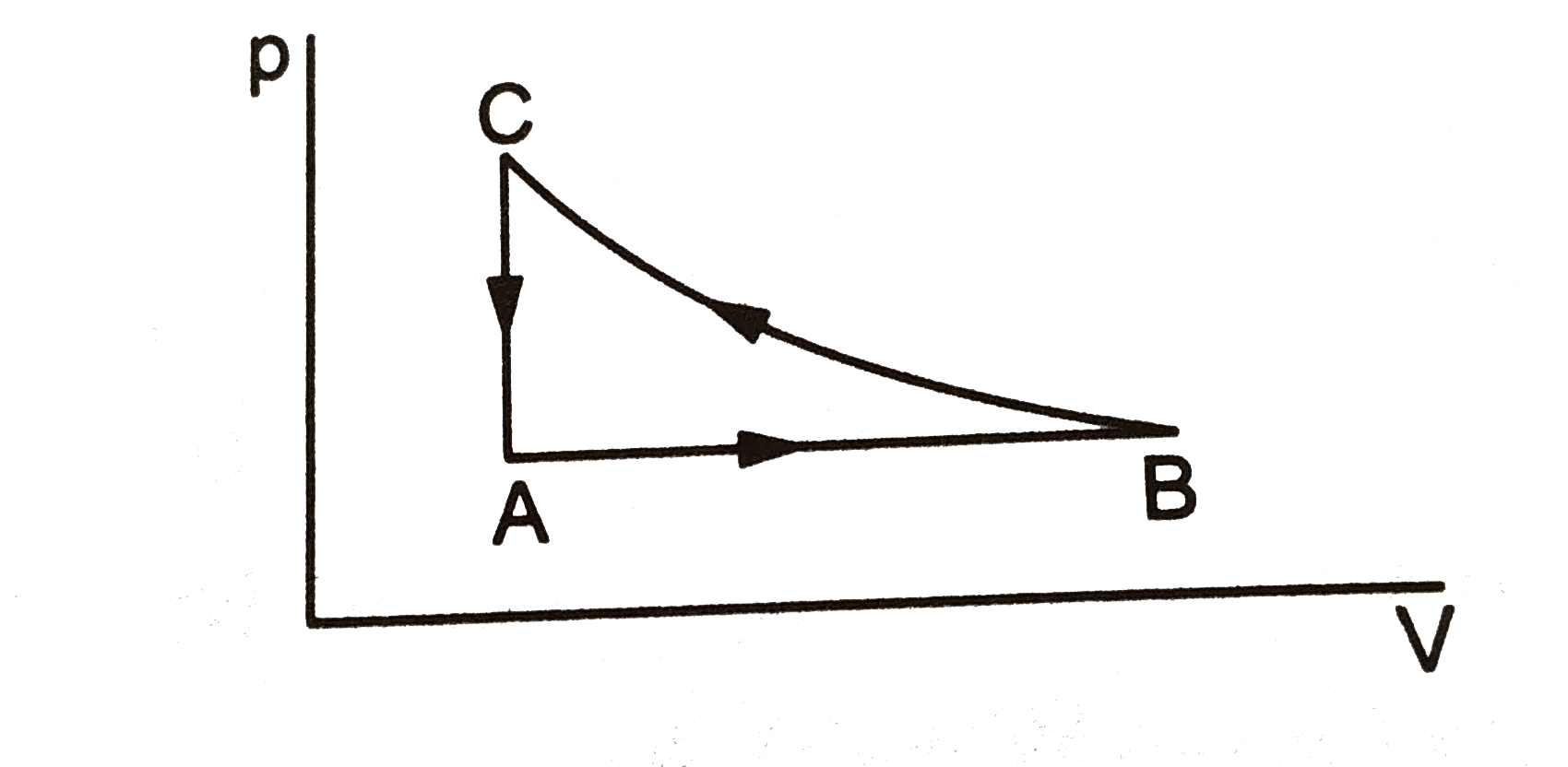
View Solutionconsider the cyclic process ABCA on a sample of 2.0 mol of an ideal gas as shown in the temperature of the gas at A and B are 300 K and 500 k respectively. A total of 1200 J heat is withdrawn from the sample in the process. Find the work done by the gas in part BC. take R=8.3JK−1mol−1
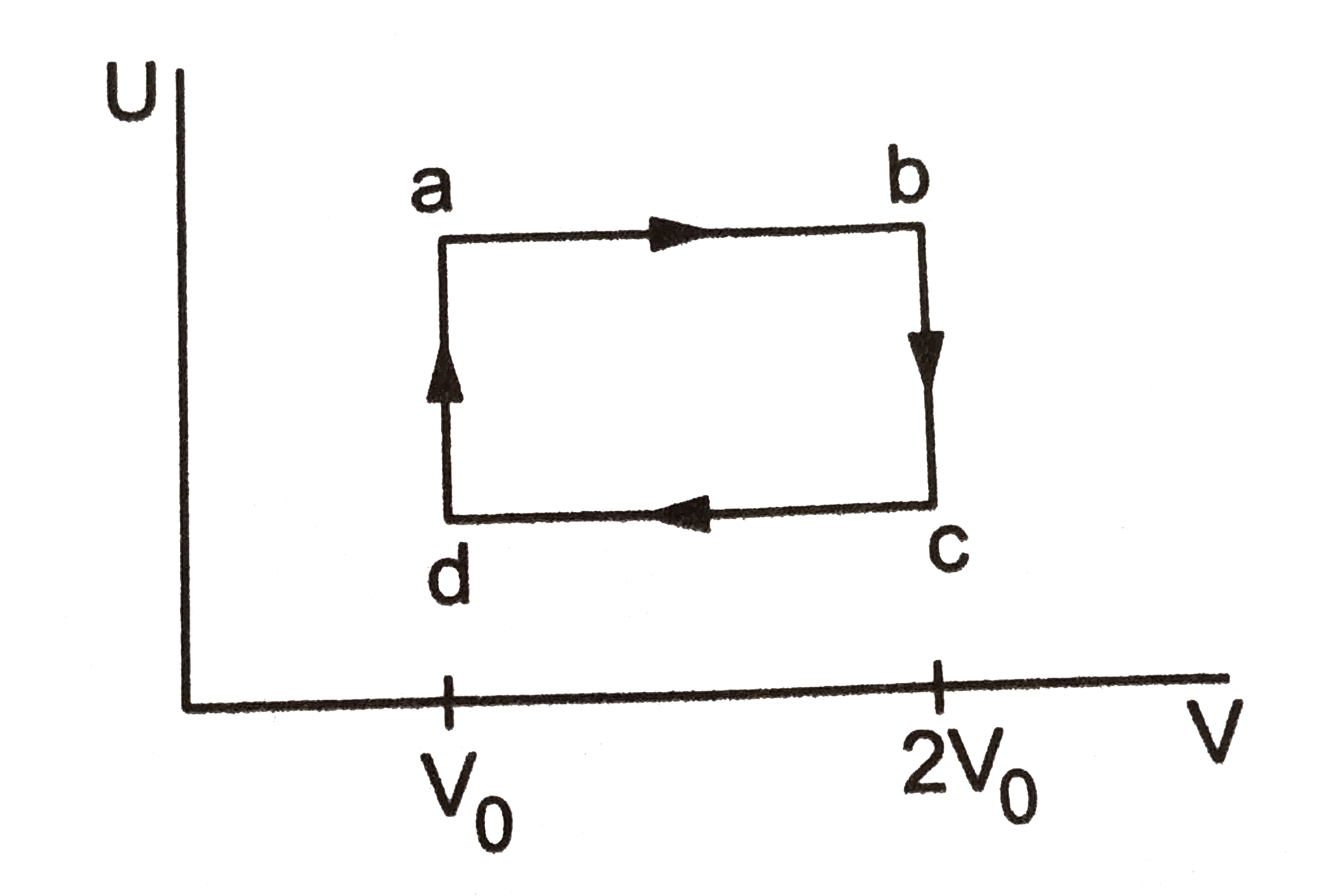
View Solutionshows the variation in the internal energy U with the volume V of 2.0 mol of an ideal gas in a cyclic process abcda. The temperatures of the gas at b and c are 500 K and 300 K respectively. Calculate the heat absorbed by the gas during the process.
View SolutionFig. shows the variation of internal energy (U) with the pressure (P) of 2.0 mole gas in cycle process abcda . The temperatures of gas at c and d are 300 and 500 K , respectively. Calculate the heat absorbed by the gas during the process.
View SolutionFigure shows the variation of internal energy (U) with the pressure (P) of 2.0 mole gas in cyclic process abcda.The temperature of gas at c and d are 300 and 500 K respectively. The heat absorbed by the gas during the process is Xxx 10 R ln 2 . Find the value of x.
View Solution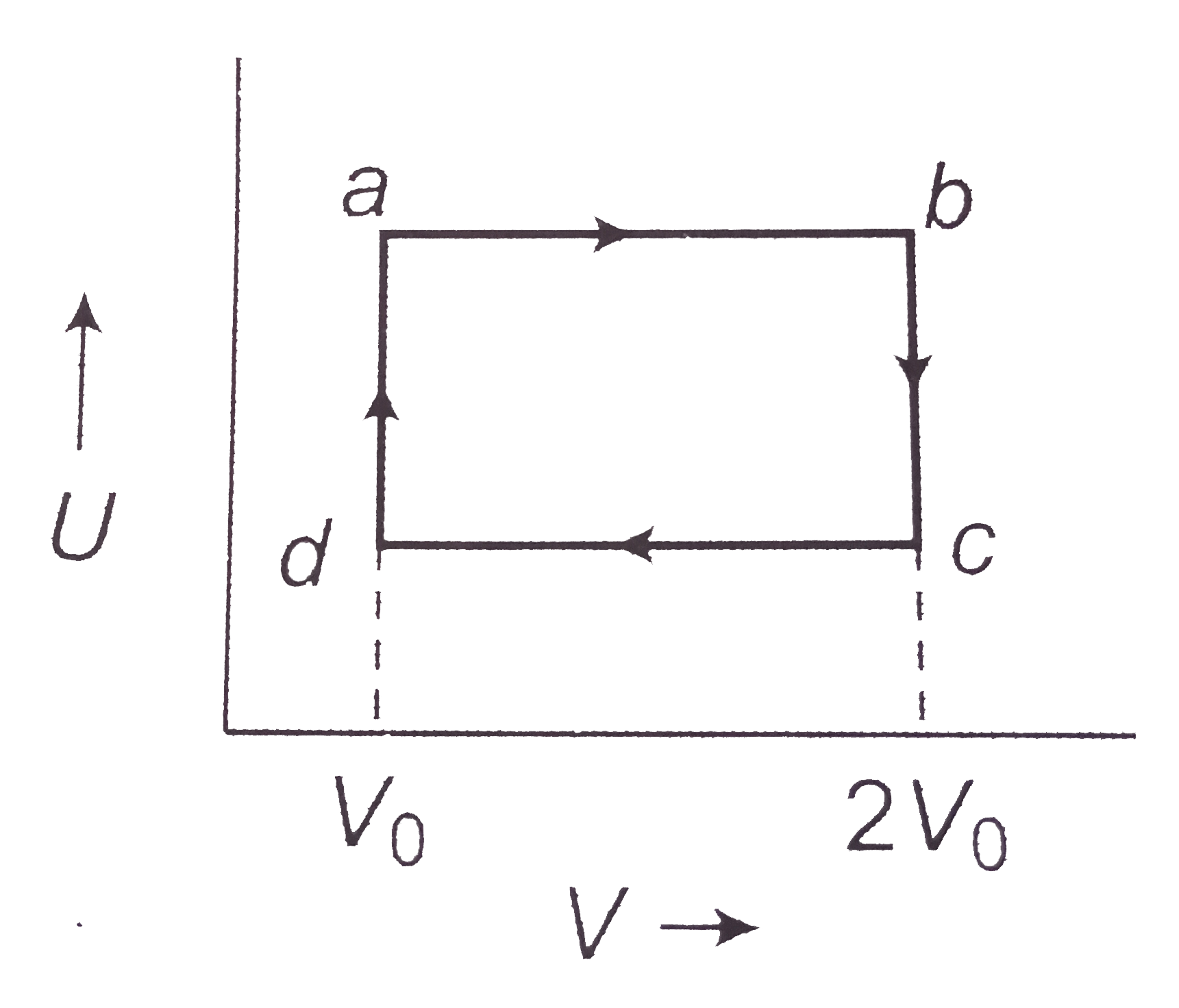 Consider the following cyclic abcda performed on 2 mole of an ideal gas. The temperature of the gas at b and c are 400 k and 300 k respectively. Calculated the heat absorbed by the gas during the process in calories.
Consider the following cyclic abcda performed on 2 mole of an ideal gas. The temperature of the gas at b and c are 400 k and 300 k respectively. Calculated the heat absorbed by the gas during the process in calories.
View Solution4 millimoles of an ideal monoatomic gas is taken through a cyclic process ABCDA, is shown in the figure, AB and CD are two isothermal curves having temperature 300 K and 600 K respectively. The total work done by the gas during the cyclic process is (xRlog_(e)2)/(5) . find the value of x .
View SolutionTwo moles of a certain ideal gas at temperature T0= 300 K were cooled isochorically so that the gas pressure reduced η= 2.0 times. Then, as a result of the isobaric process, the gas expanded till its temperature get back to the initial value. Find the total amount of heat absorbed by the gas in this process.
View SolutionOne mole of an ideal monoatomic gas undergoes a cyclic process, as shown in the figure. If the temperature of the gas at state 1 is 300 K and at state 4 is 500 K, then heat exchanged during process 2→3, is
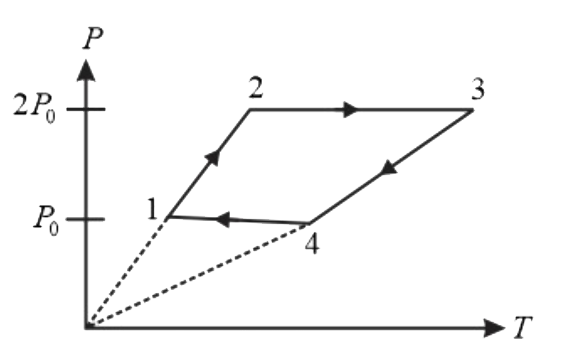
View SolutionOne mole of an ideal monoatomic gas undergoes a cyclic process, as shown in the figure. If the temperature of the gas at state 1 is 300 K and at state 4 is 500 K, then heat exchanged during process 2rarr3 , is
View SolutionFigure shows the variation of internal energy (U) with the pressure (P) of 2.0mole gas in cyclic process abeda. The temperature of gas at c and d are 300 and 500 K. How much will be the heat absorbed by the gas during the process?
View SolutionFig shows the variation in the internal energy U with the volume V of 2.0 mol of an ideal gas in a cyclic process abcda. The temperatures of the gas at b and c are 500 K and 300 K respectively. Calculate the heat absorbed by the gas during the process.

View Solution
