An ideal gas is taken from the state A (pressure P, volume V) to the state B (pressure P2 , volume 2V) along a straight line path in the P-V diagram. Select the correct statements(s) from the following.
The work done by the gas in the process A to B is negative
In the T-V diagram, the path AB becomes a part of a parabola
In the P-T diagram, the path AB becomes a part of a hyperbola
In going from A to B, the temperature T of the gas first increases to a maximum value and then decreases
The correct Answer is:B, D
Topper's Solved these Questions
- THERMODYNAMICSBOOK - AAKASH SERIESCHAPTER - THERMODYNAMICSEXERCISE - ADDITIONAL PRACTICE EXERCISE (PRACTICE SHEET ((ADVANCED) INTEGER/SUBJECTIVE TYPE QUESTIONS))
11 Videos
- THERMODYNAMICSBOOK - AAKASH SERIESCHAPTER - THERMODYNAMICSEXERCISE - PROBLEMS
42 Videos
- THERMODYNAMICSBOOK - AAKASH SERIESCHAPTER - THERMODYNAMICSEXERCISE - ADDITIONAL PRACTICE EXERCISE (PRACTICE SHEET ((ADVANCED) STRAIGHT OBJECTIVE TYPE QUESTIONS))
6 Videos
- THERMAL PROPERTIES OF MATTERBOOK - AAKASH SERIESCHAPTER - THERMAL PROPERTIES OF MATTEREXERCISE - ADDITIONAL PRACTICE EXERCISE (LEVEL - II) PRACTICE SHEET (ADVANCED) Integer/Subjective Type Questions
2 Videos
- THERMOMETRY BOOK - AAKASH SERIESCHAPTER - THERMOMETRY EXERCISE - Numerical Exercise (LEVEL- 1)
11 Videos
Similar Questions
An ideal gas is taken from the state A (pressure p, volume V) to the state B (pressure p2, volume 2V) along a straight line path in the p-V diagram. Select the correct statement(s) from the following.
View SolutionAn ideal gas is taken from the state A (pressure p, volume V) to the state B (pressure p2, volume 2V) along a straight line path in the p-V diagram. Select the correct statement(s) from the following.
View Solution
Knowledge Check
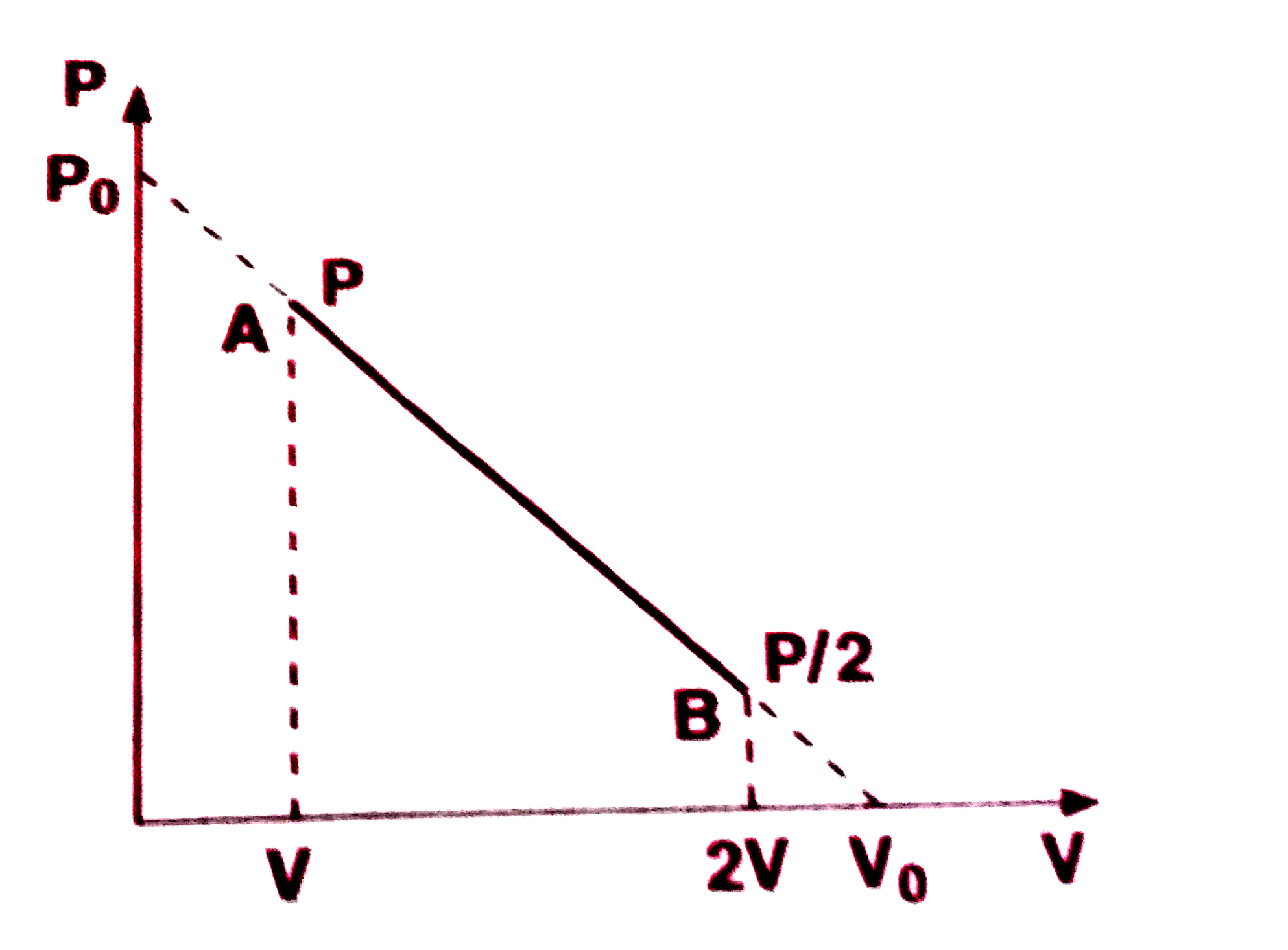
An ideal gas is taken from the state A (pressure P, volume V) to the state B (pressure p/2, volume 2V) along a straight line path in the P-V diagram. Select the correct statement (s) from the following :
AThe work done by the gas in the process A to B exceeds the work that would be done by it if the system were taken from A to B along the isotherm.BIn the T-V diagram, the path AB becomes a part of a parabolaCIn the P-T diagram, the path AB becomes a part of a hyperbolaDIn going from A to B, the temperature T of the gas first increases to a maximum value and then decreases.An ideal gas is taken from the state A (pressure p, volume V) to the state B (pressure p2, volume 2V) along a straight line path in the p-V diagram. Select the correct statement(s) from the following.
A(a) The work done by the gas in the process A to B is negativeB(b) In the T-V diagram, the path AB becomes a part of parabolaC(c) In the p-T diagram, the path AB becomes a part of a hyperbolaD(d) In going from A to B, the temperature T of the gas first increases to a maximum value and then decreases.An ideal gas is taken from the state A (pressure P, volume V) to the state B (pressure P/2, volume 2V) along a straight line path in the P-V diagram. Select the wrong statement from the following:
 AThe work done by the gas in the process A to B exceeds the work that would be done by if the system were taken from A to B along the isotherm.BIn the T-V diagram, the path AB becomes a part of parabola.CIn the P-T diagram, the path AB becomes a part of hyperbola.DIn going from A to B, the temperature T of the gas first increases to a maximum value and then decreases.
AThe work done by the gas in the process A to B exceeds the work that would be done by if the system were taken from A to B along the isotherm.BIn the T-V diagram, the path AB becomes a part of parabola.CIn the P-T diagram, the path AB becomes a part of hyperbola.DIn going from A to B, the temperature T of the gas first increases to a maximum value and then decreases.
An ideal gass is taken from state A (Pressuer P, VolumeV) to the state B
( Pressure P/2, Volume 2V) along a stright line path in PV diagram as
shown in the adjacent figure .
Select the correct statement (s) among the following .
View SolutionAn ideal gas is taken form the state A(P,V) to the state B(P2,2V) along a straight line path in the P−V diagram.
Select the correct options
(i) The work done by the gas in the process A to B exceeds the work that would be done by it if the system were taken from A to B along the isotherm
(ii) In the T−V diagram, the path AB becomes part of a hyperbola
(iii) In the P−T diagram, the path AB becomes part of a hyperbola
(iv) In going from A to B, the temperature T of the first increases to maximum value and then decreases
View SolutionAn ideal gas taken from state A(P,V) to the state B(P/2,2V) along a straight path in PV diagram. Select the correct option(s).
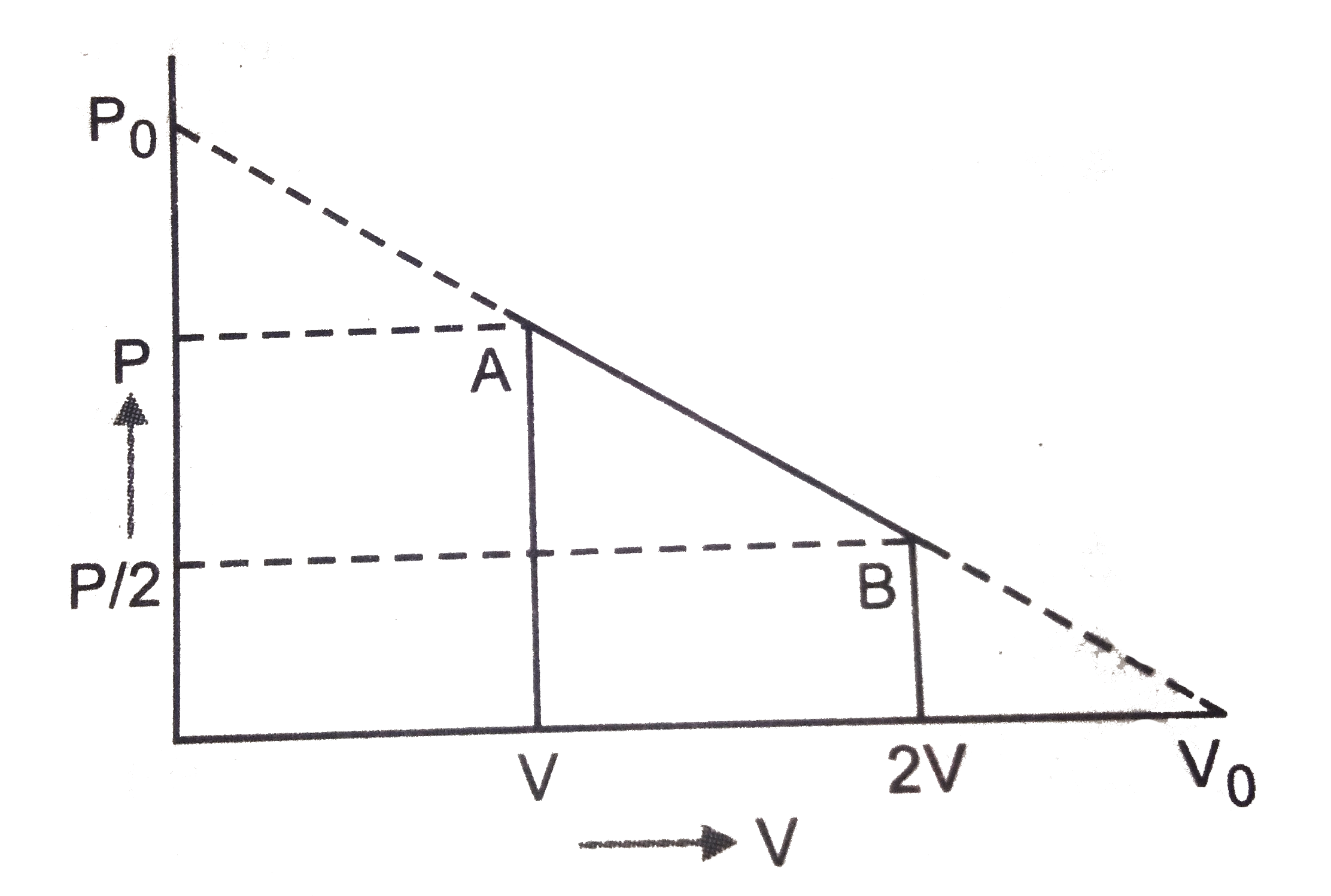
View SolutionAn ideal gas is taken from the state A (P, V) to the state B (P/2,2V) along a st. line path as shown in Fig. Select the correct statement from the following:
View Solution2 moles of ideal monatomic gas is carried from a state (P0,V0) to a state (2P0,2V0) along a straight line path in a P- V diagram. The amount of heat absorbed by
View Solution

