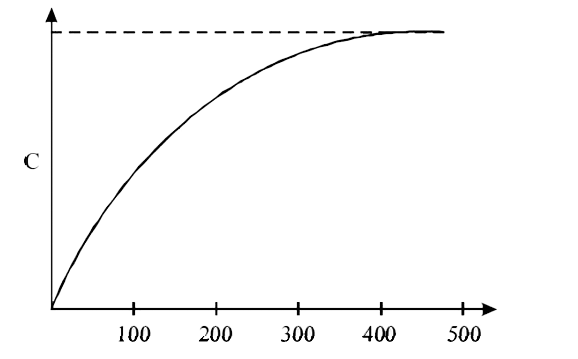
The figure shows the variation of specific heat capacity (c) of a solid as a function of temperature (T). The temperature is increased continuously from 0 to 500 K at constant rate. ignoring any volume change, the following statement(s) is (are) correct to reasonable approximation.
The rate at which heat is absorbed in the range 0 - 100K varies linearly with temperature T
Heat absorbed in increasing the temperature from 0-100K is less than the heat required for increasing the temperature from 400-500K
There is no change in the rate of heat absorption in the range 400-500K
The rate of heat absorption increase in the range 200-300K
The correct Answer is:B, C, D
Topper's Solved these Questions
- CALORIMETRY Book:AAKASH SERIESChapter:CALORIMETRY Exercise:ADDITIONAL PRACTICE EXERCISE (LEVEL - II) (LECTURE SHEET (ADVANCED)(Linked Comprehension Type Questions)
Explore 9 Videos
- CALORIMETRY Book:AAKASH SERIESChapter:CALORIMETRY Exercise:ADDITIONAL PRACTICE EXERCISE (LEVEL - II) (LECTURE SHEET (ADVANCED)(Matrix Type Questions)
Explore 1 Video
- CALORIMETRY Book:AAKASH SERIESChapter:CALORIMETRY Exercise:ADDITIONAL PRACTICE EXERCISE (LEVEL - II) (LECTURE SHEET (ADVANCED)(Straight Objective Type Questions)
Explore 19 Videos
- APPENDICES Book:AAKASH SERIESChapter:APPENDICES Exercise:GRAVITATION (EARTH SATELLITES AND ESCAPE SPEED)
Explore 35 Videos
- CENTRE OF MASSBook:AAKASH SERIESChapter:CENTRE OF MASSExercise:ADDITIONAL PRACTICE EXERCISE (PRACTICE SHEET (ADVANCED))
Explore 3 Videos
Similar Questions
An ice cube of mass 0.1 kg of 0∘C is placed in an isolated container which is 227∘C The specific heat capacity S of the container varies with temperature T according to the empirical relation S = A+BT, where A = 100 cal/kg -K and B=2×102cal/kg−K−2. If the final temperature of the container is 27∘C Latent heat of fusion of water is 8x104 cal/kg, specific heat of water is 10−3 cal/kg-k.
Heat gained by the ice will be :
View SolutionThree liquids A,B and C having same specific heat and mass m , 2m and 3m have temperature 20∘C,40∘Cand60∘C respectively. Temperature of the mixture when :
Column -IColumn -IIA) A and B are mixed,is atP)35∘CB) A and C are mixed , is at Q)52∘CC) B and C are mixed , is at Q)50∘CD) A , B and C all three are mixed , is at S)45∘CT) None
View SolutionA thermally insulated vessel contains some water at 0∘C The vessel is connected to a vacuum pump to pump out water vapour. This results in some water getting frozen. It is given latent heat of vapourisation of water at 0∘C=21×105 J/kg and latent heat of freezing of water =3.36×105 J/kg. The maximum percentage amount of water vapour that will be solidified in this manner will be
View SolutionTwo earth satellites of same mass are revolving round the earth in circular orbits of radii 6400km and 19,200 km from the centre of the earth. Ratio of their orbital velocities will be:
View SolutionA man (m1) with a bag (m2) in hand falls freely vertically from a height h above the ground. After falling through a height y0, man throws bag horizontally with a velocity u0 towards his right.
View SolutionThe figure below shows the variation of specific heat capacity (C) of a solid as a function of temperature (T). The temperature is increased continuousely form 0 to 500K at a constant rate. Ignorign any volume change, the following statement (s) is (are) correct to a reasonable approximation.
View SolutionThe specific heat of solids at low temperatures varies with absolute temperature T according to the relation S=AT3, where A is a constant. The heat energy required to raise the temperature of a mass m of such a solid from T=0 to T=20K is:
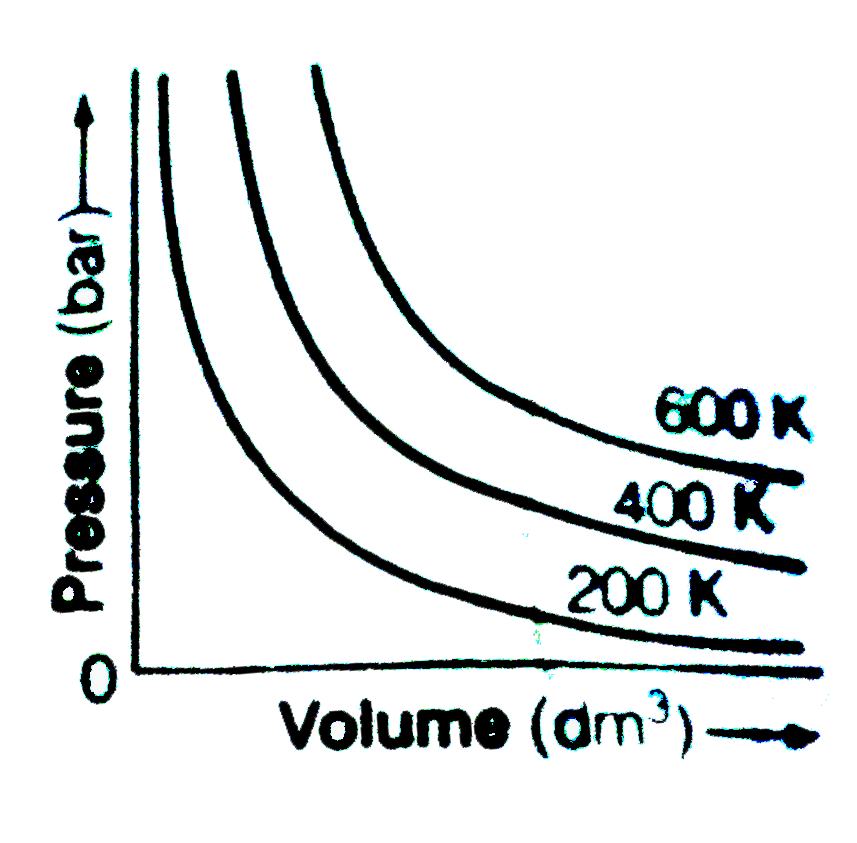
View SolutionThe variation of pressure with volume of the gas at different temperatures can be graphically represented as shown in figure. On the basis of this graph answer the following question.
(i) How will the volume of a gas change if its pressure is increased at constant temperature ?
(ii) At a constant pressure, how will the volume of a gas change if the temperature is increased from 200 K to 400 K ?
View SolutionA substance is in the solid from at 0^(@)C . The amount of heat added to this substance and its temperature is plotted in the graph . The specific heat capacity of the solid substance is 0.5 "cal"//"g"^(@)C . Specific heat capacity in the liquid state is -
View SolutionThe temperature of 2 moles of a gas is changed from 20∘C to 30∘C when heated at constant volume. If the molar heat capacity at constant volume is 8 J mol−1K−1, the change in internal energy is
View SolutionThe ratio of specific heat capacity at constant pressure to the specific heat capacity at constant volume of a diatomic gas decreases with increases in temperature . Explain
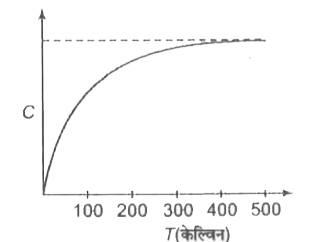
View Solutionचित्र में किसी ठोस की विशिष्ट उष्माधारिता (C ) का तापमान (T) पर निर्भरता को दर्शाया गया है | तापमान में 0 से 500 केल्विन तक समान दर से सतत वृद्धि होती है | यह मानकर कि आयतन में परिवर्तन उपेक्षणीय है, निम्न प्रकथन में कौन - सा (से) तर्कसंगत सन्निकट सही है (हैं) ?
View SolutionFor a body of mass m and specific heat capacity s, how much heat is required to increase its temperature by t?
View SolutionThe variation of pressure with volume of the gas at different temperatures can be graphically represented as shown in figure. On the basis of this graph answer the following question. (i) How will the volume of a gas change if its pressure is increased at constant temperature ? (ii) At a constant pressure, how will the volume of a gas change if the temperature is increased from 200 K to 400 K ?
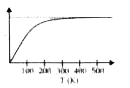
View SolutionThe figure shows the variation of specific heat capacity (C ) of a solid as a function of temperature (T). The temperature is increased continuously from 0 to 500 K at a constant rate. Ignoring any volume change, the following statements(s) is/are correct to a reasonable approximation.
View Solution

