In which of the following reactions H2O2 does not act as an reducing agent ?
A
I2+H2O2+2OH−→2I−+2H2O+O2
B
PbS+4H2O2→PbSO4+4H2O
C
2KMnO4+3H2O2→2MnO2+2KOH+2H2O+3O2
D
HOCl+H2O2→H3O++Cl−+O2
Text Solution
The correct Answer is:B
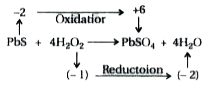
Oxygen of H2O2 (-1) is coverted to (-2) of H2O. There is reduction. H2O2 is oxidising agent.
(A), (B), (D) : H2O2 ↓2(−1)→O2↑2(O)+2e...Oxidation
|
Similar Questions
Knowledge Check
In which of the following reactions, H2O2 is acting as a reducing agent?
ASO2+H2O2→H2SO4B2KI+H2O2→2KOH+I2CPbS+4H2O2→PbSO4+4H2ODAg2O+H2O2→2Ag+H2O+O2In which of the following reactions, H2O2 is acting as a reducing agent
AH2O2+SO2→H2SO4B2KI+H2O2→2KOH+I2CPbS+4H2O2→PbSO4+4H2ODAg2O+H2O2→2Ag+H2O+O2In which of the following reactions, H2O2 is acting as a reducing agent?
APbS+4H2O2→PbSO4+4H2OB2KI+H2O2→2KOH+I2CH2O2+SO2→H2SO4DAg2O+H2O2→2Ag+H2O+O2
In which of the following reactions, H2O2 is acting as a reducing agent?
View SolutionIn which of the following reactions, H2O2 is acting as a reducing agent?
View SolutionH2O2 is a powerful oxidising agent. It is an electron acceptor in acidic as well as in alkaline medium. It can also act as reducing agent towards oxidising agents. In alkaline medium, the reducing nature of H2O2 is even more effective
In which of the following reactions H2O2 acts as a reducing agent?
View SolutionIn which of the following reaction does H2O2 acts as a reducing agent?
View SolutionIn which of the following reactions, H2O2 acts as a reducing reagent?
View Solution

