Aim: To study the reaction between calcium oxide and water.
Activity:
Take some quick lime (Calcium oxide - CaO) in a beaker. Add water to it slowly.
Touch the beaker as shown in the figure 1.3.
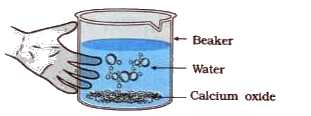
What is formed by reaction of quick lime with water ? Write reaction.Solution in Gujrati
The reaction between quick lime and water forms Ca(OH)2
CaO(s)+H2O(I)→Ca(OH)2(aq)
Similar Questions
Aim: To study the reaction between calcium oxide and water.
Activity:
Take some quick lime (Calcium oxide - CaO) in a beaker. Add water to it slowly.
Touch the beaker as shown in the figure 1.3.

What is slaked lime ?
View SolutionState the type of reaction in Adding water to quick lime to form slaked lime.
View SolutionApparatus: Test tube, glass rod, beaker, Bunsen burner, etc. Chemicals: Hydrochloric acid, ammonia solution, magnesium strip, quick lime, etc. Activity 3: Take water in a beaker up to half of its capacity. Add a few pieces of quick lime (calcium oxide, CaO) to it. What will you observe?
View SolutionWater is added to quick lime in a beaker and you touch the bottom of the beaker what do you notice ? What is the reason ?
View SolutionMention one use of calcium oxide ( lime or quick lime ).
View Solutionजब हम कैल्सियम ऑक्साइड (बिना ब्रझा हुआ चूना) में पानी डालते हैं तो होने वाली अभिक्रिया है
View SolutionWhen quick lime (CaO) is mixed with water reaction is
View Solution• Take a small amount of calcium oxide or quick lime in beaker. • Slowly add water to this. • Touch the beaker as shown in Fig. Do you feel any change in temperature?
View Solution