Aim: To study double displacement reaction between barium chloride and sodium sulphate.
Activity:
Take about 3 mL of sodium sulphate solution in a test tube.
In another test tube, take about 3 mL of barium chloride solution.
Mix the two solutions as shown in the figure.
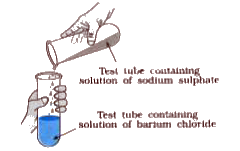
What is the colour of sodium sulphate and barium chloride solutions ?
Text Solution
Sodium sulphate and barium chloride are colourless solutions.
|
Similar Questions
Knowledge Check
Reaction between barium chloride and sodium sulphate goes to completion bacause
Abarium sulphate is almost insolubleBthe reaciton is reversibleCthe solubility of barium chloride decreasesDthe reaction is irreversibleReaction between barium chloride and aluminium sulphate is an example of
Aneutralization reactionBdisplacement reactionCprecipitation reactionDcombination reactionReaction between barium chloride and aluminium sulphate is an example of
Aneutralisation reactionBdisplacement reactionCprecipitation reaction.Dcombination reaction.
Give an example of a double displacement reaction.
View SolutionWhat happens when Sodium Sulphate reacts with Barium Chloride solution.
View SolutionState the observation for each of the following:
An aqueous solution of sodium sulphate is added to barium chloride solution.
View SolutionWrite the equation for the reaction of magnesium sulphate solution with barium chloride solution.
View Solution_____ reaction occurs when sodium sulphate reacts with barium chloride.
View Solution
