Which of the following haloalkanes is most reactive?
1-Chloropropane
1-bromopropane
2-chloropropane
2-bromopropane
The correct Answer is:D
To determine which haloalkane is the most reactive among the given options, we need to analyze the stability of the carbocations formed during the reaction and the leaving group ability of the halogens involved.
1. Identify the Haloalkanes: The given haloalkanes are:
- Chloropropane (1-chloropropane)
- Bromopropane (1-bromopropane)
- 2-Chloropropane
- 2-Bromopropane
2. Understand Carbocation Formation: When a haloalkane reacts, the halogen atom (Cl or Br) leaves, forming a carbocation. The stability of the carbocation is crucial for the reactivity of the haloalkane.
- Primary carbocation: Formed from 1-chloropropane and 1-bromopropane.
- Secondary carbocation: Formed from 2-chloropropane and 2-bromopropane.
3. Stability of Carbocations:
- Secondary carbocations are more stable than primary carbocations due to hyperconjugation and inductive effects.
- Therefore, both 2-chloropropane and 2-bromopropane will be more reactive than 1-chloropropane and 1-bromopropane.
4. Evaluate the Leaving Groups:
- The leaving group ability is also important. Bromine (Br) is a better leaving group than chlorine (Cl) because it is less electronegative and can stabilize the negative charge better when it leaves.
- Thus, 2-bromopropane will be more reactive than 2-chloropropane.
5. Conclusion:
- Among the options, 2-bromopropane is the most reactive haloalkane because it forms a stable secondary carbocation and has bromine as a better leaving group compared to chlorine.
Final Answer:
2-Bromopropane is the most reactive haloalkane among the given options.
---
- Chloropropane (1-chloropropane)
- Bromopropane (1-bromopropane)
- 2-Chloropropane
- 2-Bromopropane
- Primary carbocation: Formed from 1-chloropropane and 1-bromopropane.
- Secondary carbocation: Formed from 2-chloropropane and 2-bromopropane.
- Secondary carbocations are more stable than primary carbocations due to hyperconjugation and inductive effects.
- Therefore, both 2-chloropropane and 2-bromopropane will be more reactive than 1-chloropropane and 1-bromopropane.
- The leaving group ability is also important. Bromine (Br) is a better leaving group than chlorine (Cl) because it is less electronegative and can stabilize the negative charge better when it leaves.
- Thus, 2-bromopropane will be more reactive than 2-chloropropane.
- Among the options, 2-bromopropane is the most reactive haloalkane because it forms a stable secondary carbocation and has bromine as a better leaving group compared to chlorine.
2-Bromopropane is the most reactive haloalkane among the given options.
Topper's Solved these Questions
Similar Questions
Knowledge Check
Which of the following alkenes is most reactive towards electrophilic addition reaction?
AH2C=CH2BCH3−CH=CH2C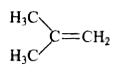 DH2C=CH−Cl
DH2C=CH−ClWhich of the following alkenes is most reactive towards cationic polymerization?
Aa. CH2=CHCH3Bb. CH2=CHClCc. CH2=CHC6H5Dd. CH2=CHCOOCH3Which of the following alkenes is the most reactive towards cationic polymerisation ?
ACH2=CHCH3BH2=CHCICH2C=CHC6H5DH2C=CHCO2CH3
Which of the following alcohols is most reactive .
View SolutionWhich of the following alkoxides is the most reactive nucleophile?
View SolutionWhich of the following phosphorus is the most reactive?
View SolutionWhich of the following halides will be most reactive in SN2 reaction and SN1 reaction respectively?
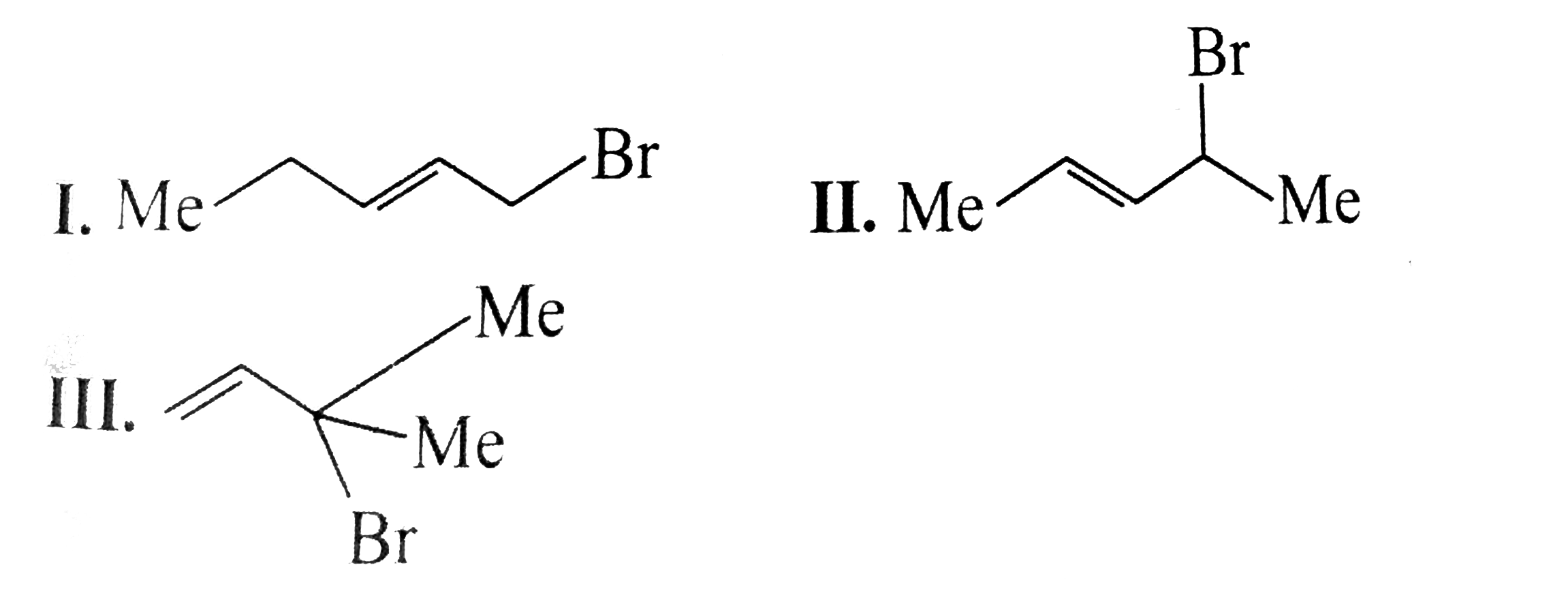
View SolutionWhich of the following compounds is the most reactive towards nucleophilic addition reactions?
View Solution