The end product Z of the reaction:
Ethyl amine HNO2−−−→XPCl5−−→YKCN−−→Z is
propanenitrile
triethylamine
diethylamine
propylamine
To solve the question step by step, we will analyze the reactions involving ethyl amine and identify the products at each stage.
Step 1: Reaction of Ethyl Amine with HNO2
Ethyl amine (C2H5NH2) reacts with nitrous acid (HNO2). This reaction is known as the carbylamine reaction, which is specific to primary amines. The product formed from this reaction is an alcohol.
Reaction:
C2H5NH2+HNO2→C2H5OH+N2+H2O
Product X:
The product X is ethyl alcohol (C2H5OH).
Step 2: Reaction of Product X with PCl5
Next, we take the product X (ethyl alcohol) and react it with phosphorus pentachloride (PCl5). This reaction converts the alcohol into an alkyl chloride.
Reaction:
C2H5OH+PCl5→C2H5Cl+POCl3+HCl
Product Y:
The product Y is ethyl chloride (C2H5Cl).
Step 3: Reaction of Product Y with KCN
Finally, we take the product Y (ethyl chloride) and react it with potassium cyanide (KCN). This reaction involves a nucleophilic substitution, where the chloride ion is replaced by the cyanide ion.
Reaction:
C2H5Cl+KCN→C2H5CN+KCl
Product Z:
The product Z is propanenitrile (C2H5CN), also known as ethyl cyanide.
Final Answer
The end product Z of the reaction is propanenitrile (C2H5CN).
---
Ethyl amine (C2H5NH2) reacts with nitrous acid (HNO2). This reaction is known as the carbylamine reaction, which is specific to primary amines. The product formed from this reaction is an alcohol.
C2H5NH2+HNO2→C2H5OH+N2+H2O
The product X is ethyl alcohol (C2H5OH).
Next, we take the product X (ethyl alcohol) and react it with phosphorus pentachloride (PCl5). This reaction converts the alcohol into an alkyl chloride.
C2H5OH+PCl5→C2H5Cl+POCl3+HCl
The product Y is ethyl chloride (C2H5Cl).
Finally, we take the product Y (ethyl chloride) and react it with potassium cyanide (KCN). This reaction involves a nucleophilic substitution, where the chloride ion is replaced by the cyanide ion.
C2H5Cl+KCN→C2H5CN+KCl
The product Z is propanenitrile (C2H5CN), also known as ethyl cyanide.
The end product Z of the reaction is propanenitrile (C2H5CN).
Topper's Solved these Questions
Similar Questions
Knowledge Check
The end product in the reaction is :
CH3CONH2P2O5−−→BH2,Ni−−−→CHNO2−−−→DCu−−−−−→300∘EACH3CHOBCH3COCH3CCH3COOC2H5D(CH3CO)2OMain product of the reaction is : CH3CONH2+HNO2→ Product
ACH3NH2BCH3CH2NH2CCH3COOHDCH3OH
The end product (Z) of the following reaction is
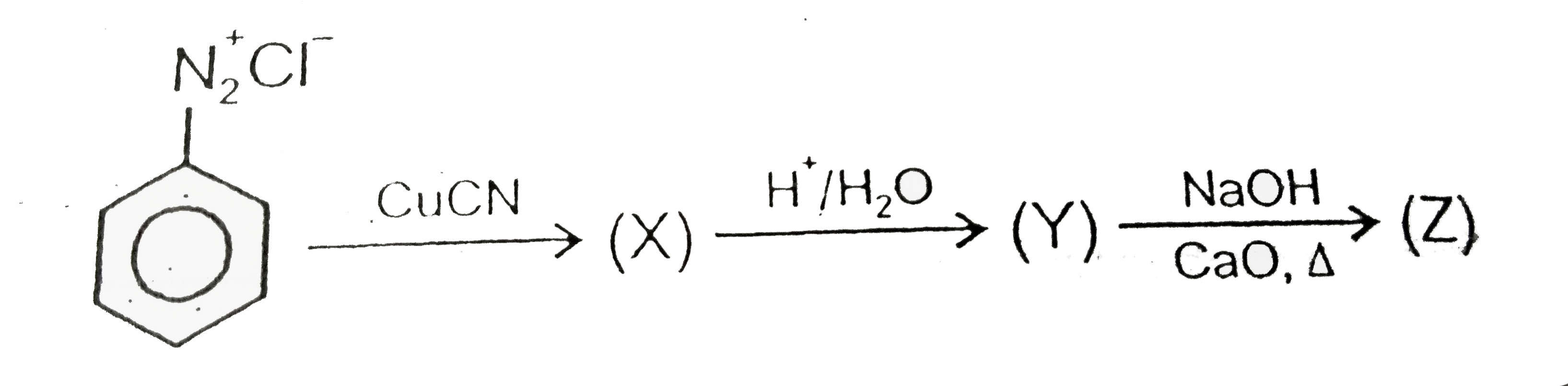
View SolutionIn the following reaction sequence ,
Ethyl amine HNO2−−−→APCl5−−→BNH3−−→C the compound C is
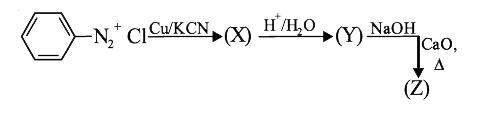
View SolutionThe end product (z ) of the following reaction is :
View SolutionWhat is the major product (Z) of the reaction?
PhCOOHHNO3−−−−−−→H2SO4Sn/HCl−−−−→NaNO2−−−−−−→HClH2O−−−−−→Δ
View SolutionThe product of the given reaction ,
CH3−∣CH3∣C∣OH−CH3∣C∣NH2−CH3HNO2−−−→ Product is :
View SolutionIdentify Z in the following reaction sequence
C2H5Ialc.KOH−−−−−→XBr2−−→YKCN−−→Z
View Solution

