Which of the following reactions of glucose can be explained only by its cyclic structure?
Glucose forms pentaacetate
Glucose reacts with hydroxylamine to form an oxime.
Pentaacetate of glucose does not react with hydroxylamine
Glucose is oxidised by nitric acid to gluconic acid.
The correct Answer is:C
To determine which reaction of glucose can be explained only by its cyclic structure, we first need to understand the structures of glucose and the reactions it undergoes.
Step 1: Draw the Structures of Glucose
1. Open Chain Structure: Glucose can exist in an open-chain form, which is an aldehyde with the formula C6H12O6. In this structure, the carbonyl group (C=O) is at the first carbon atom, and there are five hydroxyl (-OH) groups attached to the other carbon atoms.
2. Cyclic Structure: Glucose predominantly exists in a cyclic form, which is formed when the hydroxyl group on the fifth carbon reacts with the carbonyl group on the first carbon, creating a hemiacetal. This results in a six-membered ring (pyranose form) with one oxygen atom in the ring.
Step 2: Analyze the Reactions
Now, let's analyze the reactions mentioned in the question:
1. Formation of Pentaacetate: This reaction can be explained by both the open-chain and cyclic structures since both forms have hydroxyl groups that can react with acetic anhydride to form pentaacetate.
2. Reaction with Hydroxylamine to Form Oxime: This reaction can also be explained by both structures. Hydroxylamine reacts with the carbonyl group in the open-chain form and the cyclic form can also provide a carbonyl group for this reaction.
3. Reaction with Hydroxylamine to Form an Acid: This reaction is specific to the cyclic structure. In the cyclic form, the carbonyl group is not available for reaction with hydroxylamine because it is involved in the ring structure. Therefore, this reaction cannot occur in the cyclic form, making it unique to the open-chain structure.
4. Oxidation by Nitric Acid to Form Gulconic Acid: This reaction can be explained by both structures as the aldehyde group in the open-chain form and the cyclic form can be oxidized to a carboxylic acid.
Conclusion
The reaction of glucose with hydroxylamine to form an acid can only be explained by its cyclic structure, as the cyclic form does not have a free carbonyl group available for reaction. Therefore, the correct answer is:
The reaction of glucose with hydroxylamine to form an acid is the one that can be explained only by its cyclic structure.
1. Open Chain Structure: Glucose can exist in an open-chain form, which is an aldehyde with the formula C6H12O6. In this structure, the carbonyl group (C=O) is at the first carbon atom, and there are five hydroxyl (-OH) groups attached to the other carbon atoms.
2. Cyclic Structure: Glucose predominantly exists in a cyclic form, which is formed when the hydroxyl group on the fifth carbon reacts with the carbonyl group on the first carbon, creating a hemiacetal. This results in a six-membered ring (pyranose form) with one oxygen atom in the ring.
Now, let's analyze the reactions mentioned in the question:
The reaction of glucose with hydroxylamine to form an acid can only be explained by its cyclic structure, as the cyclic form does not have a free carbonyl group available for reaction. Therefore, the correct answer is:
Topper's Solved these Questions
- BIOMOLECULESBook:NCERT FINGERTPS ENGLISHChapter:BIOMOLECULESExercise:ASSERTION & REASON
Explore 15 Videos
- BIOMOLECULESBook:NCERT FINGERTPS ENGLISHChapter:BIOMOLECULESExercise:Carbohydrates
Explore 35 Videos
- BIOMOLECULESBook:NCERT FINGERTPS ENGLISHChapter:BIOMOLECULESExercise:HOTS
Explore 5 Videos
- AMINES Book:NCERT FINGERTPS ENGLISHChapter:AMINES Exercise:ASSERTION & REASON CORNER
Explore 14 Videos
- CHEMICAL KINETICSBook:NCERT FINGERTPS ENGLISHChapter:CHEMICAL KINETICSExercise:Assertion And Reason
Explore 15 Videos
Similar Questions
Knowledge Check
Which of the following sequence of reactions can give
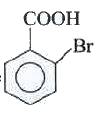 A
A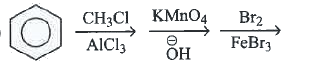 B
B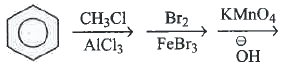 C
C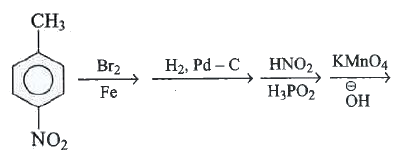 DPhMgBrCO2−−−−−→H⊕/H2OBr2AlBr3
DPhMgBrCO2−−−−−→H⊕/H2OBr2AlBr3Which of the following reactions can be used to prepare lactones (cyclic esters)?
A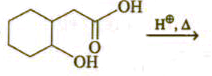 B
B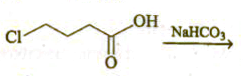 C
C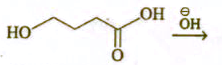 D
D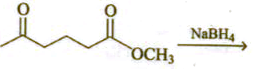
By which of the following reactions can methylcyanide be prepared?
ACH3BrKCN−−−−−→DMFBCH3NH2+CHCI3KOH−−→CCH3−CH=N−OHP2O5−−−−−→ΔDCH3−O∣∣C−NH2P4O10−−−−−→Δ
Which of the following reactions can be used to prepare methane?
View SolutionWrite the reactions of D-glucose which can't be explained by its open chain structure. How can cyclic structure of glucose explain these reactions?
View SolutionWhich of the following is a cyclic oxoacid
View SolutionEnumerate the reactions of D-Glucose which cannot be explained by its open-chain structure.
View SolutionWhich of the following reactions can be used to prepare acetophenone ?
View Solution
