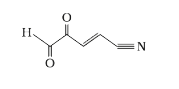
Which type of hybridisation is shown by carbon atoms from left to right in the given compound :
CH2=CH−C≡N?
sp2,sp2,sp
sp2,sp,sp
sp,sp2,sp
sp3,sp2,sp
The correct Answer is:A
To determine the type of hybridization shown by the carbon atoms in the compound CH2=CH−C≡N, we will follow these steps:
Step 1: Draw the Structure
First, we need to draw the structure of the compound clearly. The compound consists of:
- A carbon-carbon double bond between the first two carbon atoms.
- A carbon-carbon single bond between the second carbon and the third carbon.
- A carbon-nitrogen triple bond between the third carbon and the nitrogen atom.
The structure can be represented as follows:
HH C∣|C|C≡N
Step 2: Identify Each Carbon Atom
Now, we will label each carbon atom in the structure:
1. The first carbon (C1) is connected to two hydrogen atoms and one carbon atom (C2) via a double bond.
2. The second carbon (C2) is connected to one hydrogen atom and two carbon atoms (C1 and C3).
3. The third carbon (C3) is connected to one carbon atom (C2) and one nitrogen atom (N) via a triple bond.
Step 3: Count Sigma Bonds for Each Carbon Atom
Next, we will count the number of sigma bonds for each carbon atom:
- C1: Forms 3 sigma bonds (2 with H and 1 with C2).
- C2: Forms 3 sigma bonds (1 with H, 1 with C1, and 1 with C3).
- C3: Forms 2 sigma bonds (1 with C2 and 1 with N).
Step 4: Determine Hybridization
To determine the hybridization of each carbon atom, we use the formula:
\( \text{Hybridization} = \text{Number of sigma bonds} + \text{Number of lone pairs} \)
- C1: 3 sigma bonds + 0 lone pairs = 3 → Hybridization: sp²
- C2: 3 sigma bonds + 0 lone pairs = 3 → Hybridization: sp²
- C3: 2 sigma bonds + 0 lone pairs = 2 → Hybridization: sp
Conclusion
From left to right, the hybridization of the carbon atoms in the compound \( CH_2=CH-C \equiv N \) is:
- C1: sp²
- C2: sp²
- C3: sp
Thus, the answer is:
sp², sp², sp
First, we need to draw the structure of the compound clearly. The compound consists of:
- A carbon-carbon double bond between the first two carbon atoms.
- A carbon-carbon single bond between the second carbon and the third carbon.
- A carbon-nitrogen triple bond between the third carbon and the nitrogen atom.
HH C∣|C|C≡N
Now, we will label each carbon atom in the structure:
1. The first carbon (C1) is connected to two hydrogen atoms and one carbon atom (C2) via a double bond.
2. The second carbon (C2) is connected to one hydrogen atom and two carbon atoms (C1 and C3).
3. The third carbon (C3) is connected to one carbon atom (C2) and one nitrogen atom (N) via a triple bond.
Next, we will count the number of sigma bonds for each carbon atom:
- C1: Forms 3 sigma bonds (2 with H and 1 with C2).
- C2: Forms 3 sigma bonds (1 with H, 1 with C1, and 1 with C3).
- C3: Forms 2 sigma bonds (1 with C2 and 1 with N).
To determine the hybridization of each carbon atom, we use the formula:
\( \text{Hybridization} = \text{Number of sigma bonds} + \text{Number of lone pairs} \)
- C2: 3 sigma bonds + 0 lone pairs = 3 → Hybridization: sp²
- C3: 2 sigma bonds + 0 lone pairs = 2 → Hybridization: sp
From left to right, the hybridization of the carbon atoms in the compound \( CH_2=CH-C \equiv N \) is:
- C1: sp²
- C2: sp²
- C3: sp
sp², sp², sp
Similar Questions
Knowledge Check
Which type of hybridisation of each carbon is there in the compound?
CH3−CH=CH−CNAsp3,sp2,sp2,spBsp3,sp2,sp2,sp3Csp3,sp2,sp3,sp3Dsp3,sp2,sp,sp3Select the response that correctly identifies the number of carbon atoms of each type of hybridization in the compound given below
H2C=C=CH−CH=OAsp3sp2sp220Bsp3sp2sp130Csp3sp2sp031Dsp3sp2sp121
Total number of sp hybridised atoms present in the given compound is CH2=CH−C≡C−CN
View SolutionWhat is the type of hybridisation of each carbon in the following compounds?
(a) CH3−CH3
(ii) (CH3)2CO
View SolutionFind the hybridization state of each carbon atoms in following compound?
CH2=C=CH−CH2−C=C−CH2−NH2
View SolutionHow many atoms are linear in the compound (CH3−CH2−C≡C−CH=CH2) ?
View SolutionSate the nature of hybridisation of each carbon atom in the following compounds ?
(a)CH3Cl (b)(CH3)2C=O (c)CH3−C≡N (d)H−O||C−NH2 (e)CH3−CH=CH−C≡N
View SolutionWhat are hybridisation states of each carbon atom in the following compounds?
CH2=C=O,CH3CH=CH2(CH3)2CO,CH2=CHCN,C6H6
View Solution