Which of the following oxidation numbers is not correctly matched ?
Cl in HClO4
Ni in [Ni(CN)6]4−=+2
P in Mg2P2O7=+6
Cr in (NH4)2Cr2O7=+6
The correct Answer is:C
To determine which oxidation number is not correctly matched, we will analyze each compound one by one and calculate the oxidation states accordingly.
1. Analyzing HClO4 (Perchloric Acid):
- Let the oxidation number of chlorine be x.
- The oxidation number of hydrogen (H) is +1 and for oxygen (O) it is −2.
- The equation for the compound is:
+1+x+(−2×4)=0
- Simplifying this gives:
+1+x−8=0⟹x−7=0⟹x=+7
- Therefore, the oxidation number of chlorine in HClO4 is +7.
2. Analyzing Ni(CN)6^4- (Nickel Complex):
- Let the oxidation number of nickel (Ni) be x.
- The oxidation number of the cyanide ion (CN) is −1.
- The total charge of the complex is −4.
- The equation is:
x+(−1×6)=−4
- Simplifying gives:
x−6=−4⟹x=+2
- Therefore, the oxidation number of nickel in Ni(CN)6^4- is +2.
3. Analyzing Mg2P2O7 (Magnesium Phosphate):
- Let the oxidation number of phosphorus (P) be x.
- The oxidation number of magnesium (Mg) is +2 and for oxygen (O) it is −2.
- The equation is:
2(+2)+2x+(−2×7)=0
- Simplifying gives:
4+2x−14=0⟹2x−10=0⟹2x=10⟹x=+5
- Therefore, the oxidation number of phosphorus in Mg2P2O7 is +5.
4. Analyzing (NH4)2Cr2O7 (Ammonium Dichromate):
- Let the oxidation number of chromium (Cr) be x.
- The oxidation number of ammonium (NH4) is +1 and for oxygen (O) it is −2.
- The equation is:
2(+1)+2x+(−2×7)=0
- Simplifying gives:
2+2x−14=0⟹2x−12=0⟹2x=12⟹x=+6
- Therefore, the oxidation number of chromium in (NH4)2Cr2O7 is +6.
Conclusion:
- The calculated oxidation states are:
- HClO4: Cl = +7
- Ni(CN)6^4-: Ni = +2
- Mg2P2O7: P = +5
- (NH4)2Cr2O7: Cr = +6
After comparing these calculated values with the provided options, we find that all the oxidation states are correctly matched except for the phosphorus in Mg2P2O7, which is stated incorrectly in the question.
Final Answer:
The oxidation number that is not correctly matched is for phosphorus in Mg2P2O7.
---
- Let the oxidation number of chlorine be x.
- The oxidation number of hydrogen (H) is +1 and for oxygen (O) it is −2.
- The equation for the compound is:
+1+x+(−2×4)=0
- Simplifying this gives:
+1+x−8=0⟹x−7=0⟹x=+7
- Therefore, the oxidation number of chlorine in HClO4 is +7.
- Let the oxidation number of nickel (Ni) be x.
- The oxidation number of the cyanide ion (CN) is −1.
- The total charge of the complex is −4.
- The equation is:
x+(−1×6)=−4
- Simplifying gives:
x−6=−4⟹x=+2
- Therefore, the oxidation number of nickel in Ni(CN)6^4- is +2.
- Let the oxidation number of phosphorus (P) be x.
- The oxidation number of magnesium (Mg) is +2 and for oxygen (O) it is −2.
- The equation is:
2(+2)+2x+(−2×7)=0
- Simplifying gives:
4+2x−14=0⟹2x−10=0⟹2x=10⟹x=+5
- Therefore, the oxidation number of phosphorus in Mg2P2O7 is +5.
- Let the oxidation number of chromium (Cr) be x.
- The oxidation number of ammonium (NH4) is +1 and for oxygen (O) it is −2.
- The equation is:
2(+1)+2x+(−2×7)=0
- Simplifying gives:
2+2x−14=0⟹2x−12=0⟹2x=12⟹x=+6
- Therefore, the oxidation number of chromium in (NH4)2Cr2O7 is +6.
- The calculated oxidation states are:
- HClO4: Cl = +7
- Ni(CN)6^4-: Ni = +2
- Mg2P2O7: P = +5
- (NH4)2Cr2O7: Cr = +6
The oxidation number that is not correctly matched is for phosphorus in Mg2P2O7.
Similar Questions
Knowledge Check
Which of the following polymers is not correctly matched ?
AFormation of dacron-Step growth polmerisationBFormation of polytetrafluoroethene- step growth polymerisationCFormation of polythene-Chain growth polymerisation in presence of benzoyl peroxideDFormation of polyacylonitrile - Chain growth polymerisation in presence of peroxideWhich of the following pairs is not matched correctly?
ANociceptors -painBMechanoreceptors-joint positionCExteroreceptors- external environmentDEnteroreceptors-internal environmentWhich of the following pairs are not correctly matched?
A B
B C
C D
D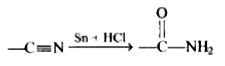
Which one of the following is correctly matched ?
View SolutionWhich of the following pairs is not correctly matched
View SolutionWhich of the following pair is not correctly matched?
View SolutionWhich of the following reactions are correctly matched ?
View SolutionWhich of the following pairs is correctly matched?
View Solution
