Which of the following reaction does not take place at cathode ?
Ag+→Ag−e−
Fe2+→Fe3++e−
Cu2++2e−→Cu
Al3+→Al−3e−
The correct Answer is:B
To determine which reaction does not take place at the cathode, we need to understand the processes occurring at the cathode and anode in an electrochemical cell.
1. Understand the Definitions:
- Oxidation: Loss of electrons.
- Reduction: Gain of electrons.
- At the anode, oxidation occurs.
- At the cathode, reduction occurs.
2. Identify the Reactions:
We need to analyze the reactions provided in the options to determine which one involves oxidation (loss of electrons).
3. Examine Each Reaction:
- Reaction 1: Ag++e−→Ag
- Here, Ag+ gains an electron to form Ag. This is a reduction reaction.
- Reaction 2: Fe2+→Fe3++e−
- In this case, Fe2+ loses an electron to become Fe3+. This is an oxidation reaction.
- Reaction 3: Cu2++2e−→Cu
- Here, Cu2+ gains electrons to form Cu. This is a reduction reaction.
- Reaction 4: Al3++3e−→Al
- In this reaction, Al3+ gains electrons to form Al. This is a reduction reaction.
4. Identify the Reaction that Does Not Occur at Cathode:
- From our analysis, Reaction 2 involves oxidation (loss of electrons) and therefore does not occur at the cathode.
5. Conclusion:
The reaction that does not take place at the cathode is Reaction 2: Fe2+→Fe3++e−.
Final Answer:
Option B: Fe2+→Fe3++e− does not take place at the cathode.
- Oxidation: Loss of electrons.
- Reduction: Gain of electrons.
- At the anode, oxidation occurs.
- At the cathode, reduction occurs.
We need to analyze the reactions provided in the options to determine which one involves oxidation (loss of electrons).
- Reaction 1: Ag++e−→Ag
- Here, Ag+ gains an electron to form Ag. This is a reduction reaction.
- Reaction 2: Fe2+→Fe3++e−
- In this case, Fe2+ loses an electron to become Fe3+. This is an oxidation reaction.
- Reaction 3: Cu2++2e−→Cu
- Here, Cu2+ gains electrons to form Cu. This is a reduction reaction.
- Reaction 4: Al3++3e−→Al
- In this reaction, Al3+ gains electrons to form Al. This is a reduction reaction.
- From our analysis, Reaction 2 involves oxidation (loss of electrons) and therefore does not occur at the cathode.
The reaction that does not take place at the cathode is Reaction 2: Fe2+→Fe3++e−.
Option B: Fe2+→Fe3++e− does not take place at the cathode.
Similar Questions
Knowledge Check
Which of the following reactions does not take place?
AC2H5Br+KNO2→C2H5−O−N=O+KBrB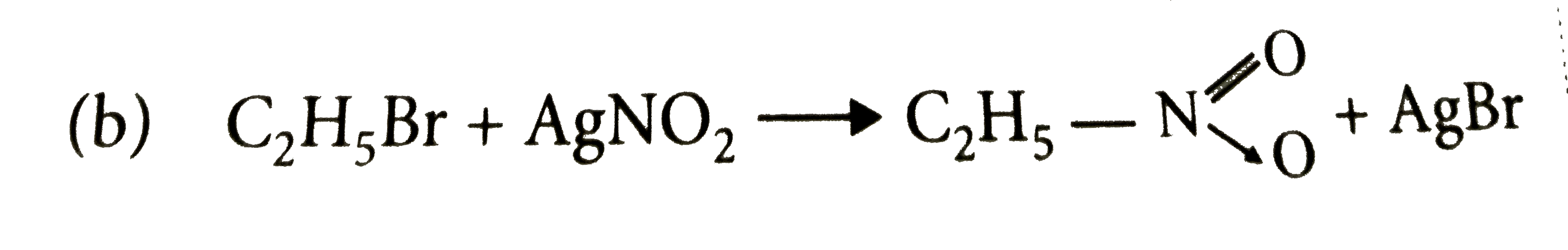 CC2H5Br+AgCN→C2H5NC+AgBrDC2H5Br+KCN→C2H5NC+KBr
CC2H5Br+AgCN→C2H5NC+AgBrDC2H5Br+KCN→C2H5NC+KBrThe light reaction does not take place in
AstromaBgranaCthylakoidsDintergranal lamellaeWhich of the following reactions takes place at anode ?
AReductionBOxidationCDecompositionDDissolution
Which of the following events does not take place after the degeneration of the generative cell in pollen grain ?
View SolutionIn which of the following , Convection of heat does not take place ?
View SolutionWhich of the following reactions occurs at the cathode?
View SolutionWhich of the following reactions does not take place during leaching for concentration of bauxite ?
View SolutionWhich of the following reactions does not take place during rusting?
View Solution

