Which of the following IUPAC names is not correctly matched?

(CH3)2C=CHCOOH : 3-Methylbut-2-enoic acid
PhCH2CH2COOH: 3-Phenylpropanoic acid
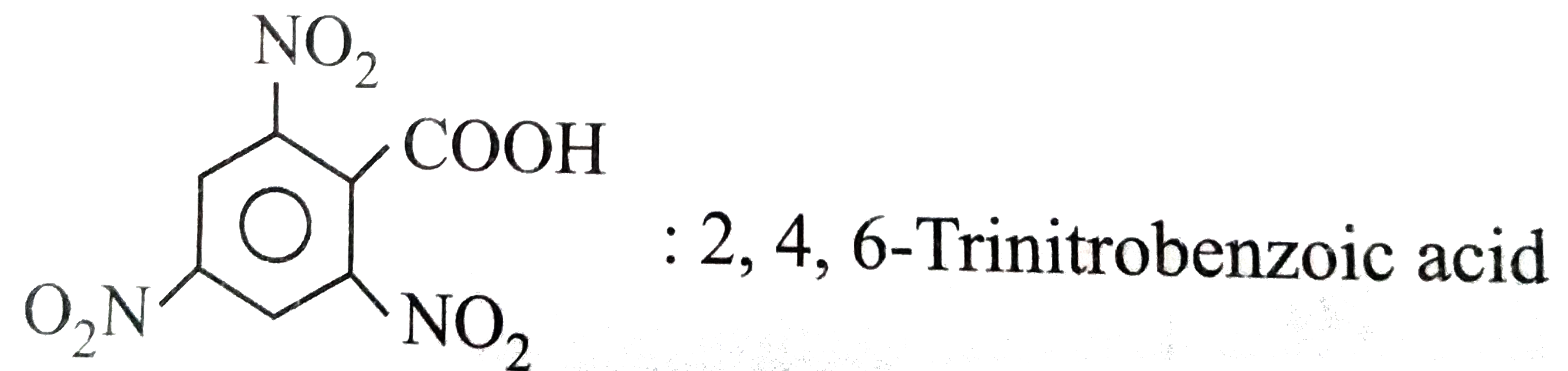
The correct Answer is:A
To determine which of the given IUPAC names is not correctly matched, we will analyze each option step by step.
Step 1: Analyze Option A
1. Structure Identification: Identify the structure of the compound. It is a cyclic compound with five carbon atoms (cyclopentane) and a carboxylic acid functional group.
2. Numbering: Start numbering from the carbon with the carboxylic acid group to give the substituents the lowest possible numbers. The numbering goes as follows:
- 1 (carboxylic acid), 2 (methyl), 3, 4, 5.
3. Substituent Identification: The substituent is a methyl group on the second carbon.
4. Naming: The name should be 2-methylpentanoic acid. However, the option does not match this name correctly.
Step 2: Analyze Option B
1. Structure Identification: This compound has a four-carbon chain (butane) with a carboxylic acid group and a methyl group.
2. Numbering: Number the chain starting from the end closest to the carboxylic acid:
- 1 (carboxylic acid), 2, 3 (methyl), 4.
3. Substituent Identification: The substituent is a methyl group on the third carbon.
4. Naming: The correct name is 3-methylbutanoic acid. This matches the given name.
Step 3: Analyze Option C
1. Structure Identification: This compound has a propane chain with a phenyl group as a substituent.
2. Numbering: Number the chain starting from the end closest to the carboxylic acid:
- 1 (carboxylic acid), 2, 3 (phenyl).
3. Substituent Identification: The substituent is a phenyl group on the third carbon.
4. Naming: The correct name is 3-phenylpropanoic acid. This matches the given name.
Step 4: Analyze Option D
1. Structure Identification: This compound has a benzene ring with three nitro groups and a carboxylic acid group.
2. Numbering: Number the benzene ring to give the nitro groups the lowest possible numbers:
- 1 (carboxylic acid), 2 (nitro), 4 (nitro), 6 (nitro).
3. Substituent Identification: The substituents are nitro groups at positions 2, 4, and 6.
4. Naming: The correct name is 2,4,6-trinitrobenzoic acid. This matches the given name.
Conclusion
After analyzing all options, it is clear that Option A is the incorrectly matched IUPAC name. The correct name should be 2-methylpentanoic acid, but it does not match the provided name.
Final Answer
Option A is not correctly matched.
---
1. Structure Identification: Identify the structure of the compound. It is a cyclic compound with five carbon atoms (cyclopentane) and a carboxylic acid functional group.
2. Numbering: Start numbering from the carbon with the carboxylic acid group to give the substituents the lowest possible numbers. The numbering goes as follows:
- 1 (carboxylic acid), 2 (methyl), 3, 4, 5.
3. Substituent Identification: The substituent is a methyl group on the second carbon.
4. Naming: The name should be 2-methylpentanoic acid. However, the option does not match this name correctly.
1. Structure Identification: This compound has a four-carbon chain (butane) with a carboxylic acid group and a methyl group.
2. Numbering: Number the chain starting from the end closest to the carboxylic acid:
- 1 (carboxylic acid), 2, 3 (methyl), 4.
3. Substituent Identification: The substituent is a methyl group on the third carbon.
4. Naming: The correct name is 3-methylbutanoic acid. This matches the given name.
1. Structure Identification: This compound has a propane chain with a phenyl group as a substituent.
2. Numbering: Number the chain starting from the end closest to the carboxylic acid:
- 1 (carboxylic acid), 2, 3 (phenyl).
3. Substituent Identification: The substituent is a phenyl group on the third carbon.
4. Naming: The correct name is 3-phenylpropanoic acid. This matches the given name.
1. Structure Identification: This compound has a benzene ring with three nitro groups and a carboxylic acid group.
2. Numbering: Number the benzene ring to give the nitro groups the lowest possible numbers:
- 1 (carboxylic acid), 2 (nitro), 4 (nitro), 6 (nitro).
3. Substituent Identification: The substituents are nitro groups at positions 2, 4, and 6.
4. Naming: The correct name is 2,4,6-trinitrobenzoic acid. This matches the given name.
After analyzing all options, it is clear that Option A is the incorrectly matched IUPAC name. The correct name should be 2-methylpentanoic acid, but it does not match the provided name.
Option A is not correctly matched.
Topper's Solved these Questions
Similar Questions
Knowledge Check
Which of the following IUPAC name is not correctly matched?
A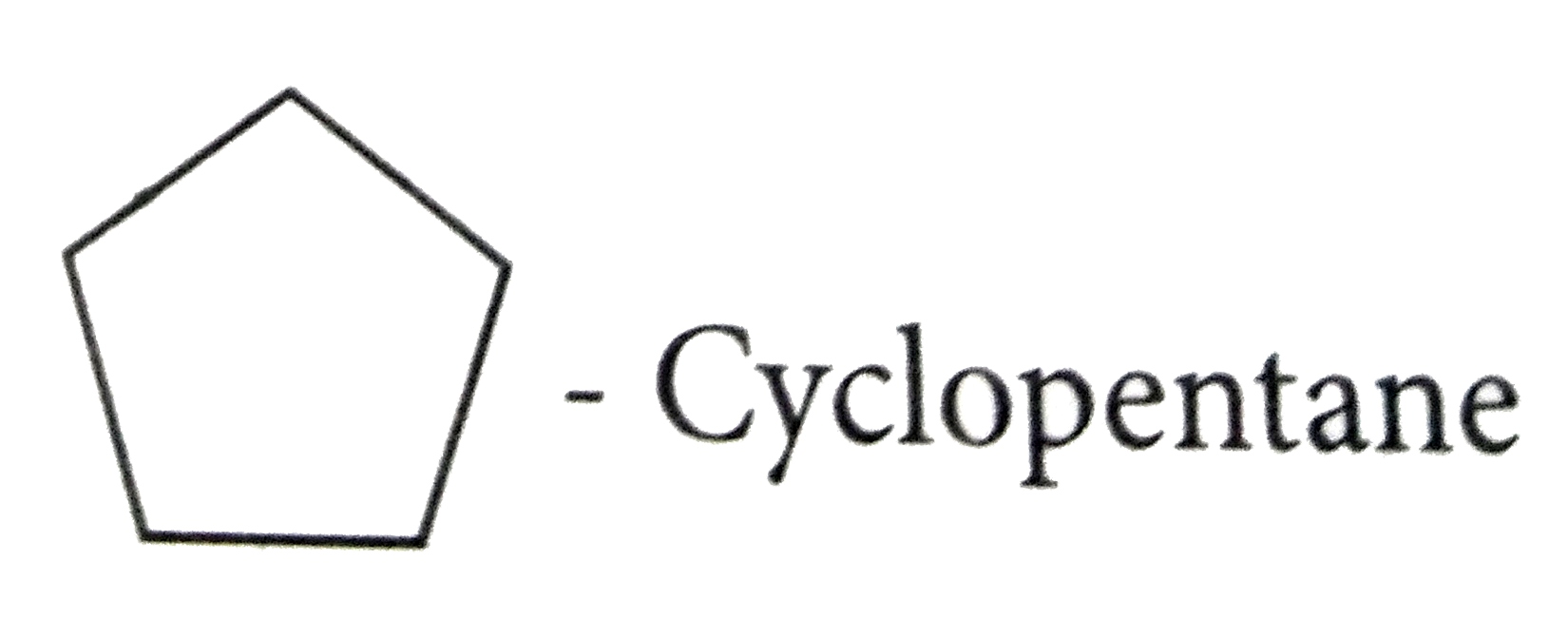 B
B C
C D
D
Which of the followingg compounds is not correctly matched wiith its IUPAC name?
ACH3CH2CH2COOCH2CH3-Ethyl butanoateBCH3−C∣CH3H−CH2−CHO−3−MethylbutanalCCH3−C∣CH3H−C∣∣O−CH2CH3−2−methylpentan-3-oneDCH3−C∣OHH−C∣CH3H−CH3−3−Methylbutan-3-olWhich of the following pairs are not correctly matched?
A B
B C
C D
D
Which of the following IUPAC name correctly matched ?
View SolutionWhich one of the following is correctly matched ?
View SolutionWhich of the following is/are not correctly matched?
View SolutionWhich of the following is not correctly matched with its IUPAC names?
View SolutionWhich of the following pairs is not matched correctly?
View Solution

