Cannizzaro's reaction is not given by _________ .

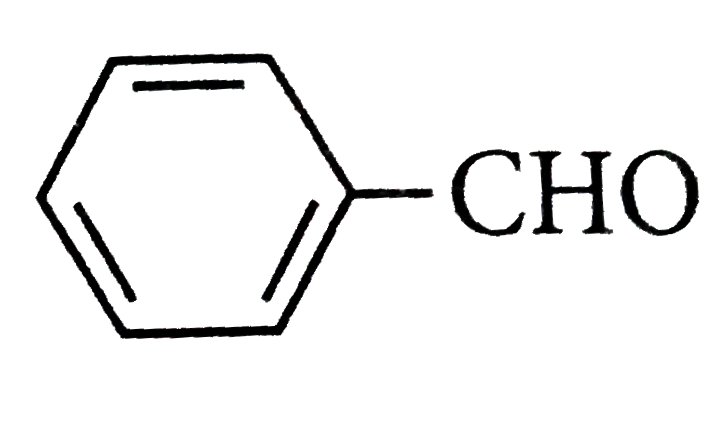
HCHO
CH3CHO
The correct Answer is:D
To answer the question "Cannizzaro's reaction is not given by _________", we need to understand the conditions under which Cannizzaro's reaction occurs and identify the aldehyde that does not participate in this reaction.
1. Understanding Cannizzaro's Reaction:
- Cannizzaro's reaction is a redox reaction that occurs in aldehydes that do not have alpha-hydrogens. In this reaction, one molecule of the aldehyde is oxidized to a carboxylic acid, while another molecule is reduced to an alcohol.
2. Identifying Aldehydes:
- Aldehydes can be classified based on the presence or absence of alpha-hydrogens. Aldehydes with at least one alpha-hydrogen will not undergo Cannizzaro's reaction.
3. Examples of Aldehydes:
- Formaldehyde (HCHO) does not have alpha-hydrogens and can undergo Cannizzaro's reaction.
- Acetaldehyde (CH3CHO) has alpha-hydrogens and cannot undergo Cannizzaro's reaction.
- Benzaldehyde (C6H5CHO) also does not have alpha-hydrogens and can undergo Cannizzaro's reaction.
- Propionaldehyde (C2H5CHO) has alpha-hydrogens and cannot undergo Cannizzaro's reaction.
4. Analyzing the Options:
- Given the options (let's assume they are A, B, C, and D), we need to determine which aldehyde does not participate in Cannizzaro's reaction.
- If one of the options is an aldehyde with alpha-hydrogens, that would be the correct answer.
5. Conclusion:
- Based on the understanding that aldehydes with alpha-hydrogens do not undergo Cannizzaro's reaction, we can conclude that the correct answer is the aldehyde that has alpha-hydrogens.
Final Answer:
Cannizzaro's reaction is not given by aldehydes that contain alpha-hydrogens, such as Acetaldehyde (if it is one of the options).
---
- Cannizzaro's reaction is a redox reaction that occurs in aldehydes that do not have alpha-hydrogens. In this reaction, one molecule of the aldehyde is oxidized to a carboxylic acid, while another molecule is reduced to an alcohol.
- Aldehydes can be classified based on the presence or absence of alpha-hydrogens. Aldehydes with at least one alpha-hydrogen will not undergo Cannizzaro's reaction.
- Formaldehyde (HCHO) does not have alpha-hydrogens and can undergo Cannizzaro's reaction.
- Acetaldehyde (CH3CHO) has alpha-hydrogens and cannot undergo Cannizzaro's reaction.
- Benzaldehyde (C6H5CHO) also does not have alpha-hydrogens and can undergo Cannizzaro's reaction.
- Propionaldehyde (C2H5CHO) has alpha-hydrogens and cannot undergo Cannizzaro's reaction.
- Given the options (let's assume they are A, B, C, and D), we need to determine which aldehyde does not participate in Cannizzaro's reaction.
- If one of the options is an aldehyde with alpha-hydrogens, that would be the correct answer.
- Based on the understanding that aldehydes with alpha-hydrogens do not undergo Cannizzaro's reaction, we can conclude that the correct answer is the aldehyde that has alpha-hydrogens.
Cannizzaro's reaction is not given by aldehydes that contain alpha-hydrogens, such as Acetaldehyde (if it is one of the options).
Topper's Solved these Questions
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:NCERT FINGERTPS ENGLISHChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:ASSERTION & REASON
Explore 15 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:NCERT FINGERTPS ENGLISHChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:Nomenclature And Structure Of Carbonyl Group
Explore 3 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:NCERT FINGERTPS ENGLISHChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:MCQs(CHEMICAL REACTIONS )
Explore 18 Videos
- ALCOHOLS,PHENOLS AND ETHERSBook:NCERT FINGERTPS ENGLISHChapter:ALCOHOLS,PHENOLS AND ETHERSExercise:Assertion And Reason
Explore 14 Videos
- AMINES Book:NCERT FINGERTPS ENGLISHChapter:AMINES Exercise:ASSERTION & REASON CORNER
Explore 14 Videos
Similar Questions
Assertion : In formaldehyde all the four atoms lie in one plane.
Reason : Carbonyl carbon forms a π-bond with oxygen by overlapping of p -orbitals.
View SolutionWhich of the following structures is not correctly matched ?
View SolutionWhich of the following compounds would have the smallest value for pKa?
View SolutionAssertion : Catalytic reduction of butanal gives butanol.
Reason: Aldehydes on reduction give corresponding primary alcohols.
View SolutionAssertion : Isopropylamine is a secondary amine .
Reason : IUPAC name of isopropylamine is 2-aminopropane .
View SolutionThe Cannizzaro's reaction is not given by
View SolutionCannizzaro's reaction is not given by
View SolutionThe Cannizzaro's reaction would be given by :
View SolutionThe Cannizzaro's reaction is not given by
View SolutionCannizzaro's reaction is not given by _________ .
View SolutionCannizzaro's reaction is
View SolutionCannizzaro's reaction is given by
View SolutionCannizzaro's reaction is not given by
View SolutionCannizzaro's reaction is not given by
View SolutionThe Cannizzaro's reaction is not given by :
View Solution
