Identify the following electromagnetic radiations as per the wavelengths given below. Write one application of each.
(a) 10−3nm (b) 10−3m (c ) 1nm
(a) γ-rays - used in medicine to destroy cancer cells.
(b) Microwaves - used for radar system for aircraft navigation.
(c) X-rays – used for studying crystal structure. (b) 103 m (c) 1 nm
Similar Questions
H-atom is exposed to electromagnetic radiation of λ=1025.6 Å and excited atom gives out induced radiation. What is the minimum wavelength of the induced radiation?
(a)102.6nm
(b)12.09 nm
(c)121.6 nm
(d)810.8 nm
View SolutionIdentify the electromagnetic waves whose wavelengths vary as
(a) 10−12m<λ<10−8m
(b) 10−3m<λ<10−1m
Write one use for each.
View Solution
Knowledge Check
Which one of the following frequency of radiation (in Hz) has a wavelength of 600 nm
A2×1013B5×1016C2×1014D5×1014A bulb is emitted electromagnetic radiation of 660 nm wave length. The Total energy of radiation is 3 x 10−18JThe number of emitted photon will be : (h=6.6×10−34J×s,C=3×108m/s)
A1B10C100D1000A substance absorbs electromagnetic radiations of wavelength 12.3 nm and then emits another electromagnetic radiations of wavelength 24.6 nm . If ratio of number of photons absorbed to number of photons emitted is 2:1 then ratio of energy absorbed to energy emitted will be :
A2:1B1:1C4:1D1:4
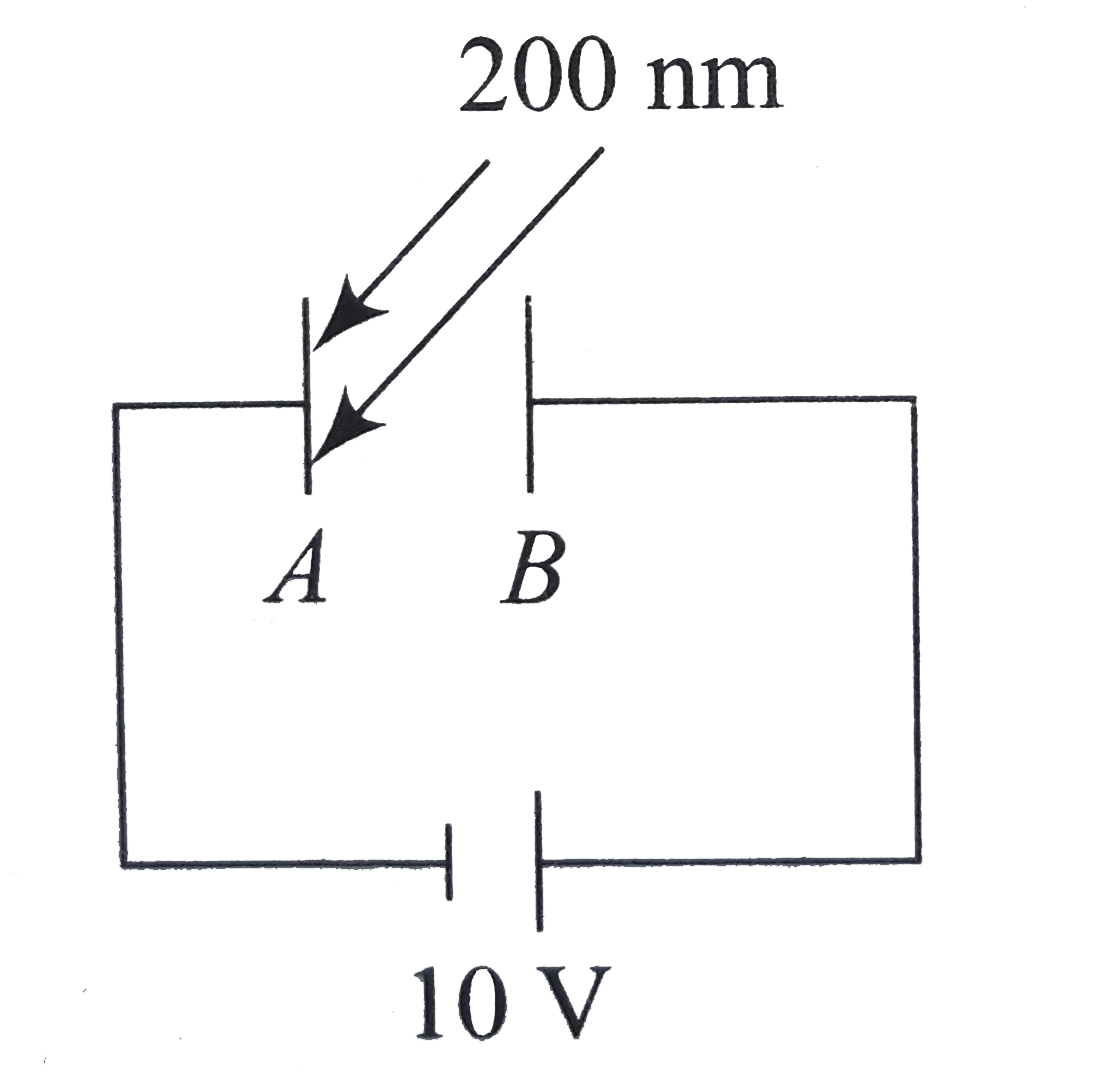
In Fig. electromagnetic radiations of wavelength 200nm are incident on a metallic plate A. the photoelectrons are accelerated by a potential difference of 10 V. These electrons strike another metal plate B from which electromagnetic radiations are emitted. The minimum wavelength of emitted photons is 100nm. If the work function of metal A is found to be (x×10−1)ev, then find the value of x. (Given hc=1240eV nm)
View Solution
In Fig. electromagnetic radiations of wavelength 200nm are incident on a metallic plate A. the photoelectrons are accelerated by a potential difference of 10 V. These electrons strike another metal plate B from which electromagnetic radiations are emitted. The minimum wavelength of emitted photons is 100nm. If the work function of metal A is found to be (×x10−1)ev, then find the value of x. (Given hc=1240eV nm)
View SolutionA bulb is emitted electromagnetic radiation of 660 nm wave length.
The Total energy of radiation is 3 x 10−18JThe number of
emitted photon will be : (h=6.6×10−34J×s,C=3×108m/s)
View SolutionIdentify the electromagnetic waves whose wavelength very as: (a) 10−12m<λ<10−8m (b) 10−3m<λ<10−1m. Write one use each.
View SolutionIdentify the electromagnetic waves whose wavelength very as: (a) 10−12m<λ<10−8m (b) 10−3m<λ<10−1m. Write one use each.
View Solution

