Resorcinol has the structure
A

B

C

D

Text Solution
The correct Answer is:B
|
Topper's Solved these Questions
- ALCOHOLS, PHENOLS AND ETHERSBOOK - U-LIKE SERIESCHAPTER - ALCOHOLS, PHENOLS AND ETHERSEXERCISE - ASSERTION-REASON QUESTIONS
10 Videos
- ALCOHOLS, PHENOLS AND ETHERSBOOK - U-LIKE SERIESCHAPTER - ALCOHOLS, PHENOLS AND ETHERSEXERCISE - FILL IN THE BLANKS
9 Videos
- ALCOHOLS, PHENOLS AND ETHERSBOOK - U-LIKE SERIESCHAPTER - ALCOHOLS, PHENOLS AND ETHERSEXERCISE - CASE BASED/SOURCE-BASED INTEGRATED QUESTIONS
14 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBOOK - U-LIKE SERIESCHAPTER - ALDEHYDES, KETONES AND CARBOXYLIC ACIDSEXERCISE - SELF ASSESSMENT TEST (SECTION B)
1 Video
Similar Questions
Knowledge Check
TNT has the structure
A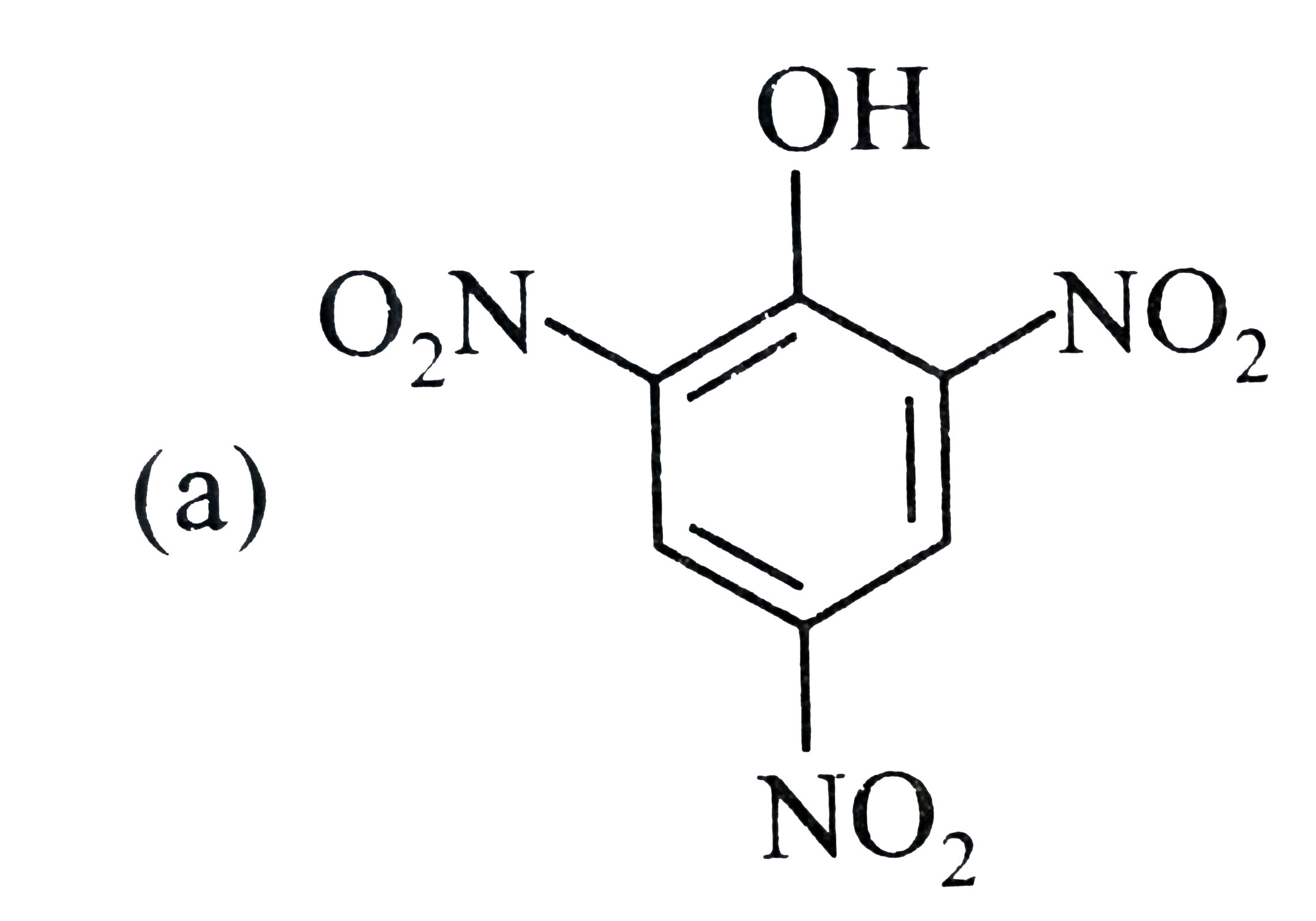 B
B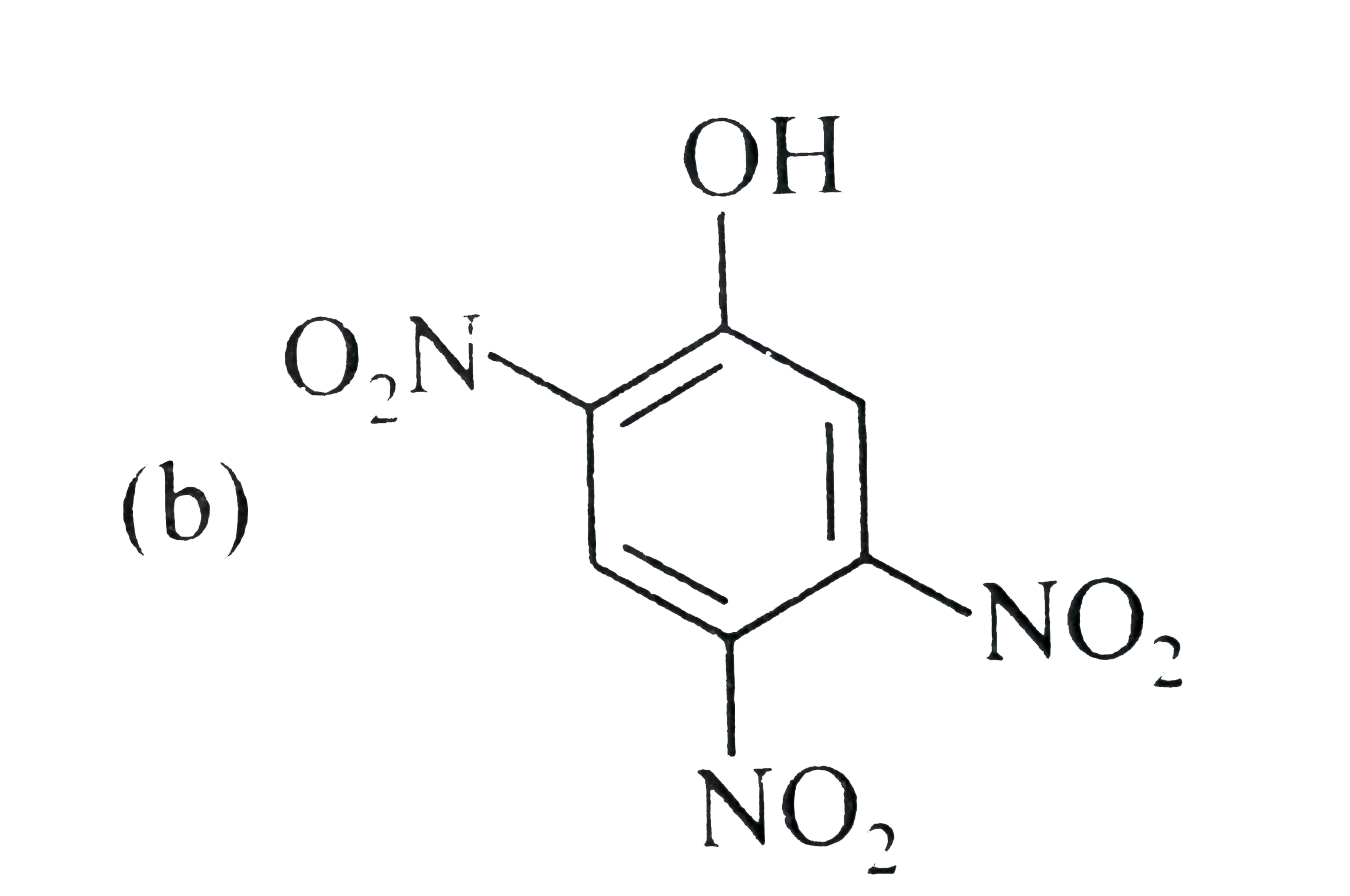 C
C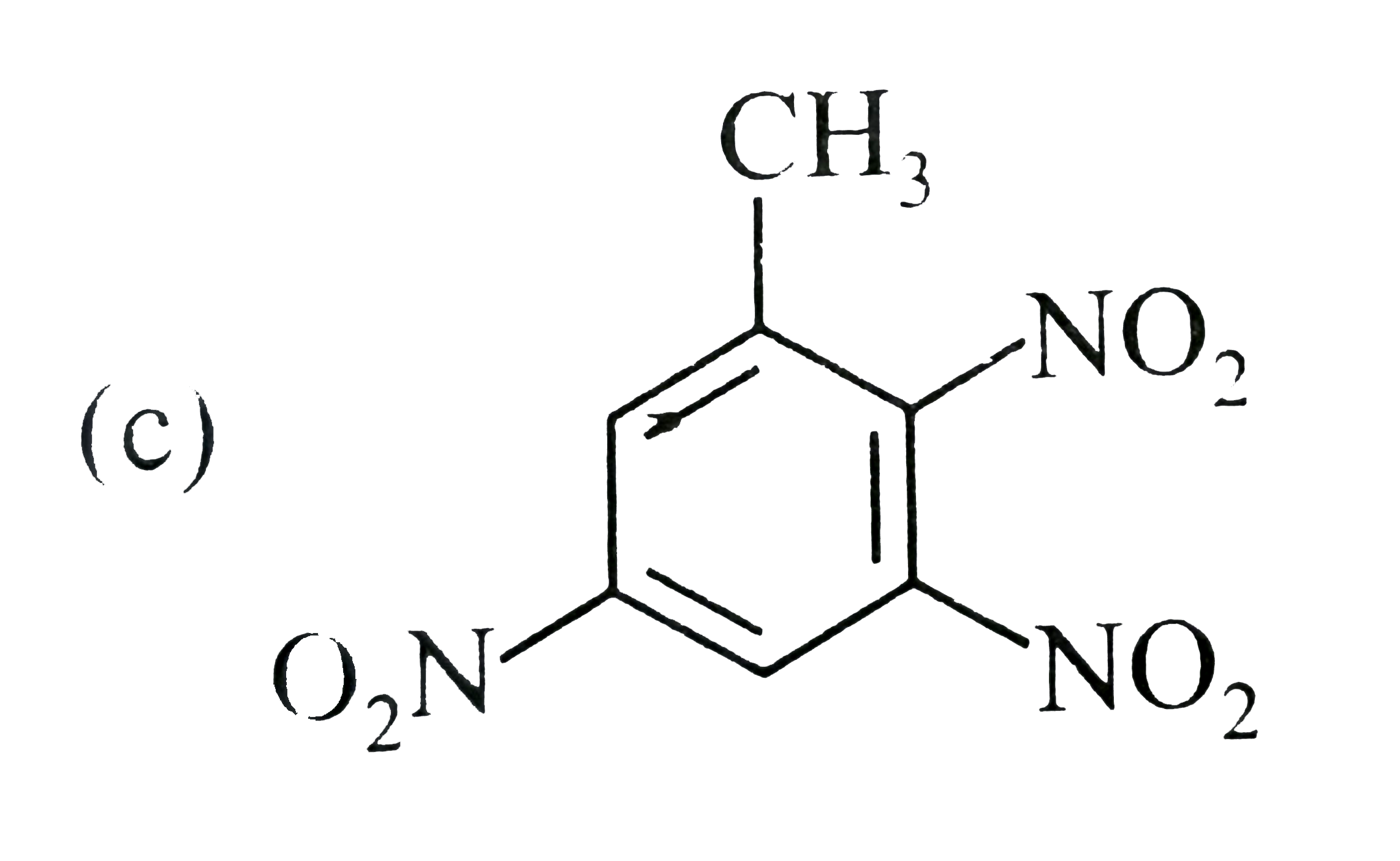 D
D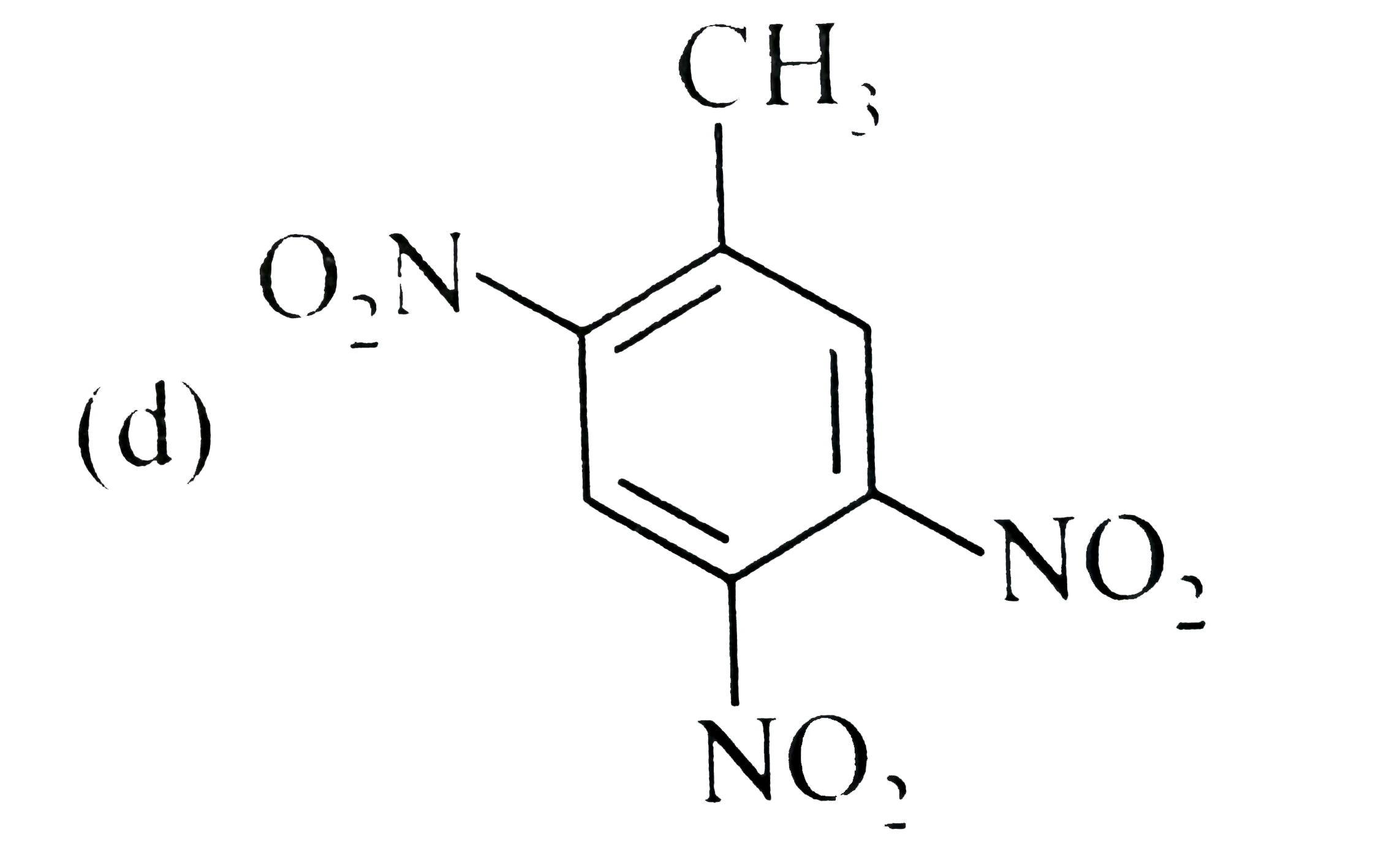
XeO3 has the structure
AsquareBsquare pyramidalCpyramidalDdistorted octahedralIn the reaction
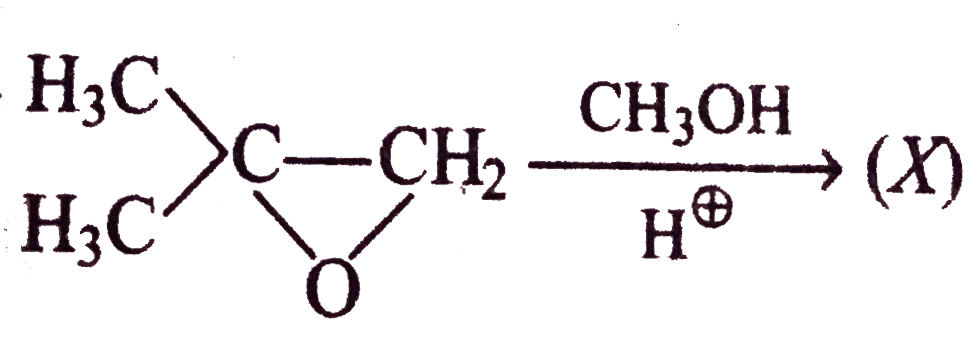
The product X has the structure"AH3C−CH3∣C∣OCH3−CH2OHBH3C−CH3∣C∣OH−CH2−OCH3CH3C−CH3∣C∣OCH3−CH3DCH3C−CH3∣C−CH2−OCH3
MgO has a structure of NaCl and TiCl has the structure of CsCl. What are the coordination number of ions in each (MgO and TiCl)
View SolutionMgO has a structure of NaCl and TiCl has the structure of CsCl. What are the coordination number of ions in each (MgO and TiCl)
View SolutionMgO has a structure of NaCl and TiCl has the structure of CsCl. What are the coordination number of ions in each (MgO and TiCl)
View SolutionMgO has the structure of NaCl and TICI has the structure of CsCl. What are the coordination number of ions in MgO and TICI ?
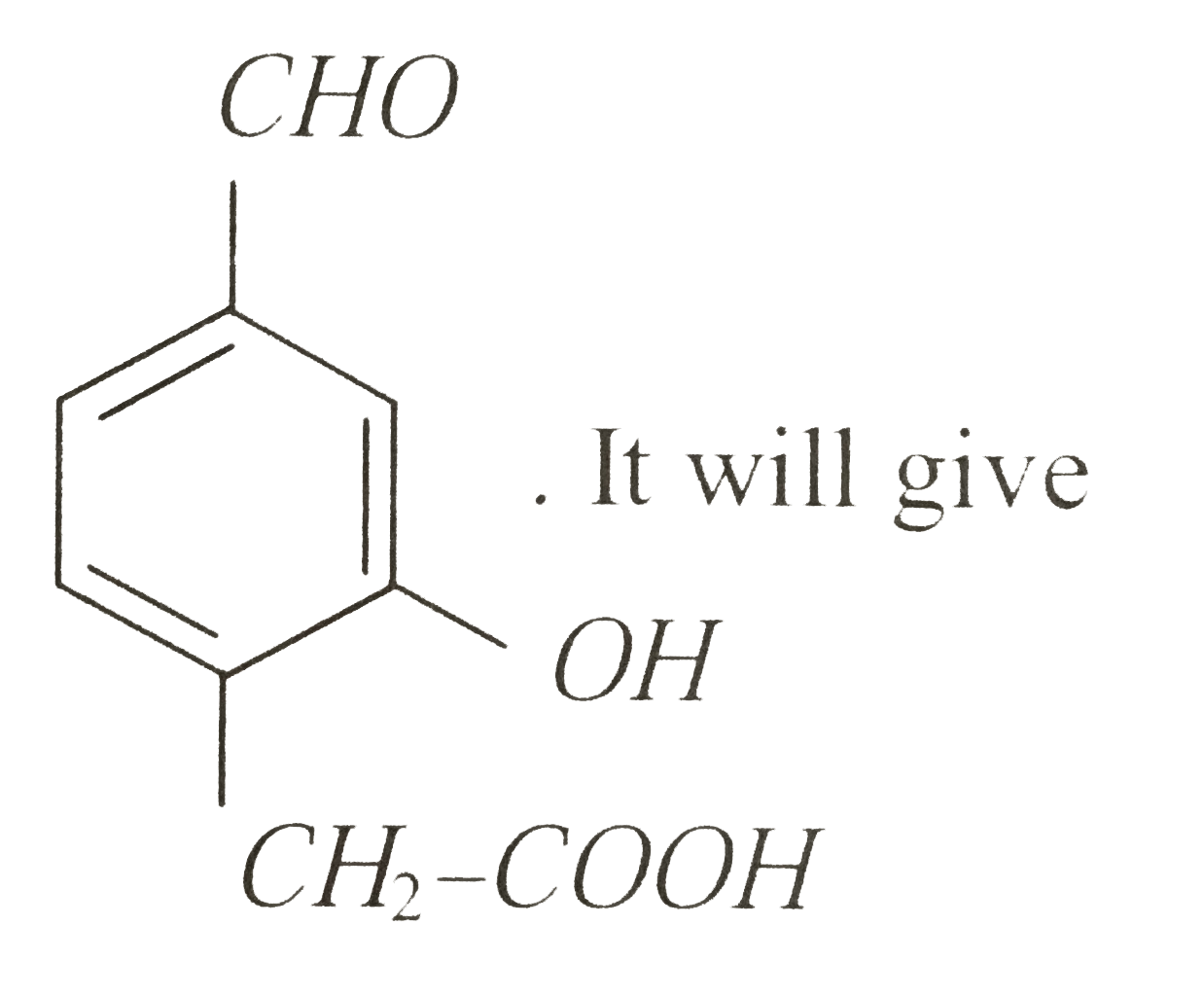
View SolutionAn organic compound has the structure
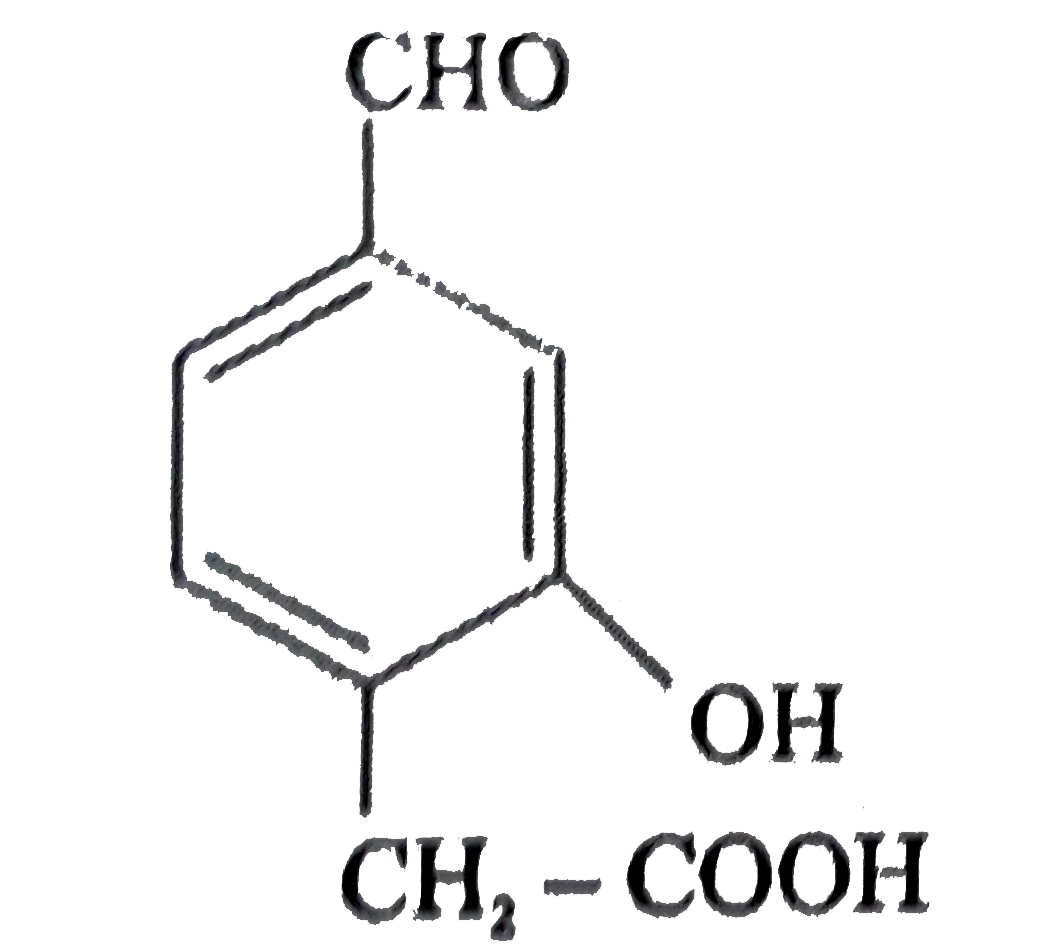
It will give
View Solution