Assertion (A) : Ethers are classified on the basis of alkyl groups attached to the oxygen atom.
Reason (R) : The presence of −OH group in phenols deactivates the aromatic ring towards electrophilic substitution.
Both Assertion (A) and Reason (R) are correct statements, and Reason (R) is the correct explanation of the Assertion (A).
Both Assertion (A) and Reason (R) are correct statements, but Reason (R) is not the correct explanationof the Assertion (A).
Assertion (A) is correct, but Reason (R) is incorrect statement.
Assertion (A) is incorrect, but Reason (R) is correct statement.
The correct Answer is:C
Similar Questions
Explain how does the −OH group attached to a carbon of benzene ring activate it towards electrophilic substitution?
View SolutionExpalin (i) the - OH group attached to aromatic eing in phenols and (ii) alkoxy group in the alkyl aryl ethers are ortho , para directing and activate the aromatic ring towards electrophilic substitution .
View Solution
Knowledge Check
Which of the following froups groups deactivates the benzene ring towards electrophilic substitution ?
A−NHRB−OHC−COORD−ORWhich of the following group activates the benzene ring most towards electrophilic substitution
A−CHOB−NR2C−NHCOCH3D−NO2Which of the following group(s) activate the benzene ring towards electrophilic substitution reaction.
A−ClB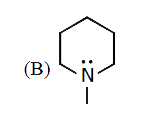 C−O−O||C−CH3D−C≡..N
C−O−O||C−CH3D−C≡..N
Meta-directing and deactivating group in the aromatic electrophilic substitution is :
View SolutionMeta-directing and deactivating group in the aromatic electrophilic substitution is :
View SolutionThe presence of which one of the following group on benzene nucleus activate it towards electrophilic substitution :
View SolutionWhich of the following groups is o-p directing but deactivates benzene ring for electrophilic substitution?
View SolutionAll the common m-directig group make the benzene ring towards electrophilic substitution reactions
View Solution

