Explain how an OH group attached to a carbon in the benzene ring activates benzene towards electrophilic substitution.
Text Solution
An −OH group activates the benzene ring towards electrophilic substitution by increasing negative charge on the ring through resonance.

Since there is −ve charge at o - and p - position, it means −OH group activates benzene ring toward electrophilic substitution reaction.
|
Similar Questions
Knowledge Check
Which of the following substituents deactivates the benzene ring towards electrophilic substitution?
A−CHOB−NR2C−NHCOCH3D−NO2Which of the following group activates the benzene ring most towards electrophilic substitution
A−CHOB−NR2C−NHCOCH3D−NO2Which of the following group(s) activate the benzene ring towards electrophilic substitution reaction.
A−ClB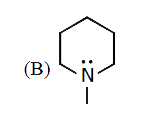 C−O−O||C−CH3D−C≡..N
C−O−O||C−CH3D−C≡..N
Explain how does the (−−−OH) group attached to a carbon of benzene ring activate it towards electophililc substitution.
View SolutionExplain the fact that in aryl ethers, (i) the alkoxy group activates the benzene ring towards electrophilic substitution and (ii) it directs the incoming substituents to ortho and para positions in benzene ring.
View Solution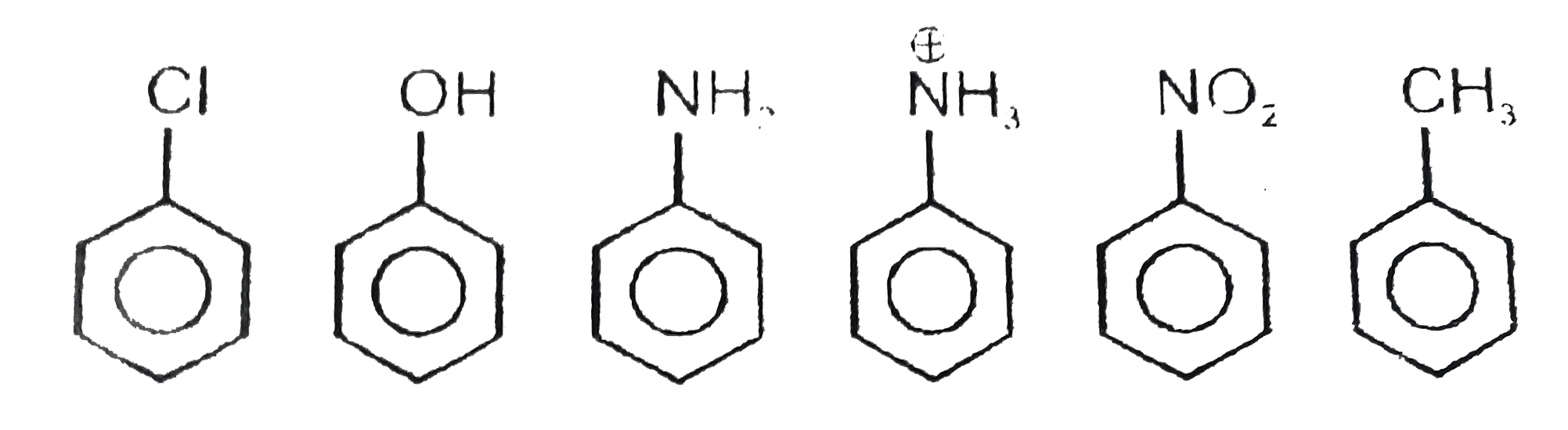
How many of the following compounds are more reactive than benzene towards electrophilic substitution.
View SolutionWhich of the following substituents deactivates the benzene ring towards electrophilic substitution?
View SolutionWhich of the following froups groups deactivates the benzene ring towards electrophilic substitution ?
View Solution

