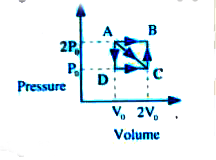
Figure shows the graph of pressure versus volume of an ideal gas, taken from state A to C via three different paths, i.e., ABC, AC and ADC. The internal energies of the gas corresponding to the states A and C are (P0V0)and(5P0V0) respectively. Determine
The change in internal energy of the gas during the process AC.
 Solution in Telugu
Solution in Telugu
Change in internal energy of the gas during the process AC=ΔU=UC−UA
=5P0V0−P0V0=4P0V0
Similar Questions
Figure shows the graph of pressure versus volume of an ideal gas, taken from state A to C via three different paths, i.e., ABC, AC and ADC. The internal energies of the gas corresponding to the states A and C are (P0V0)and(5P0V0) respectively. Determine
The total heat energy supplied to the system, during each Pressure of three processes ABC,AC and ADC.
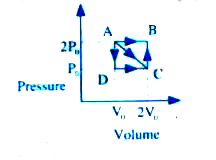
View SolutionFigure shows the graph of pressure versus volume of an ideal gas, taken from state A to C via three different paths, i.e., ABC, AC and ADC. The internal energies of the gas corresponding to the states A and C are (P_0 V_0) and (5P_0 V_0) respectively. Determine Equation of the straight line AC.
View SolutionIn the given graph, an ideal gas changes its state from A to C by two paths ABC and AC.
(a) Find the path along which work done is less.
(b) The internal energy of gas at A is 10J and the amount of heat supplied in path AC is 200J. Calculate the internal energy of gas at C.
(c) The internal energy of gas at state B is 20J. Find the amount of heat supplied to the gas to go from A to B.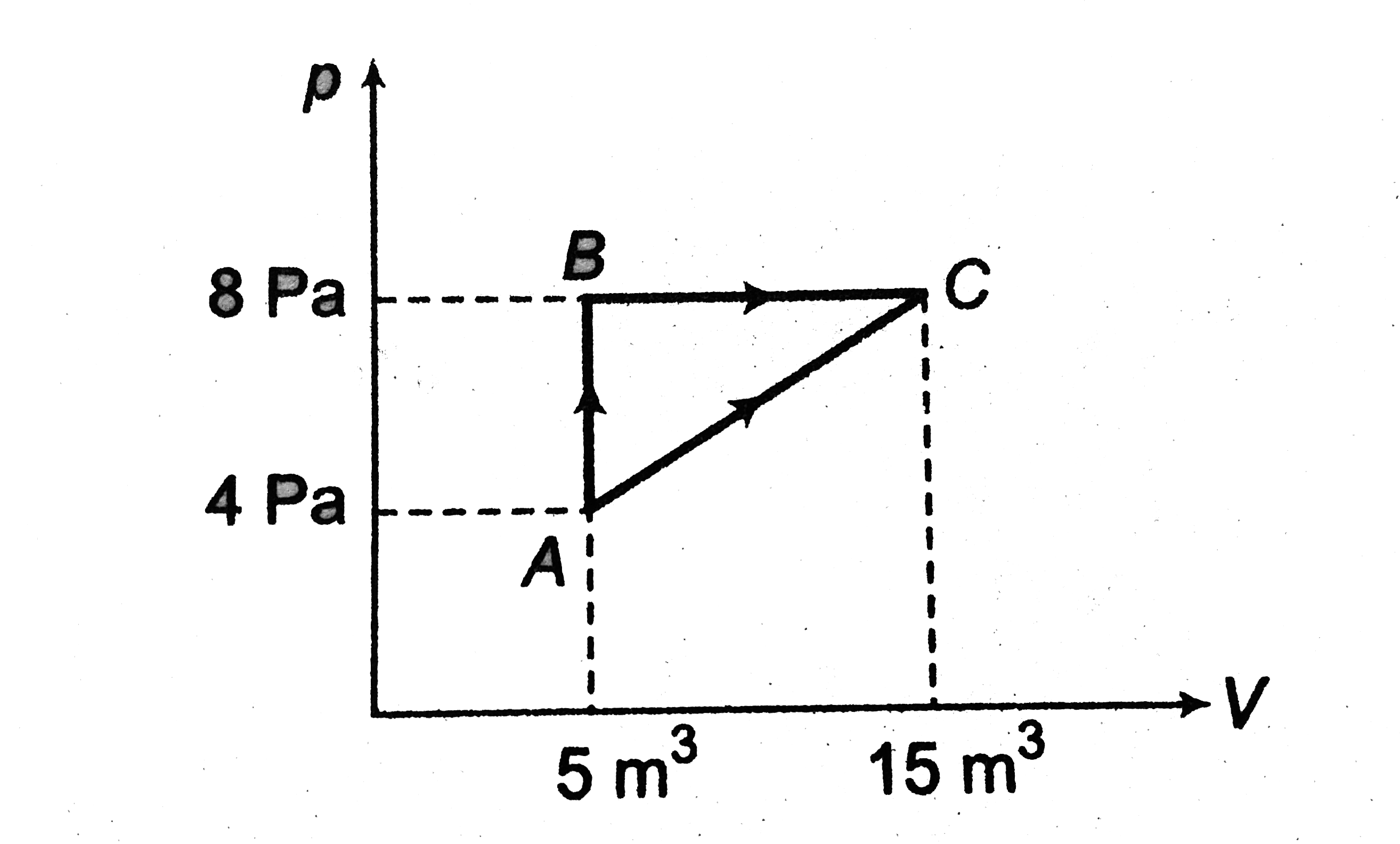
View SolutionTwo moles of a monoatomic gas are taken from a to c, via three paths abc, ac and adc. Work done by the gas in process ac is
View SolutionFigure shows an ideal gas changing its state A to state C by two different path ABC and AC. The internal energy of the gas at A is 10J and the amount of heat supplied to change its state to C through the path AC is 200J. Find the internal energy at C.
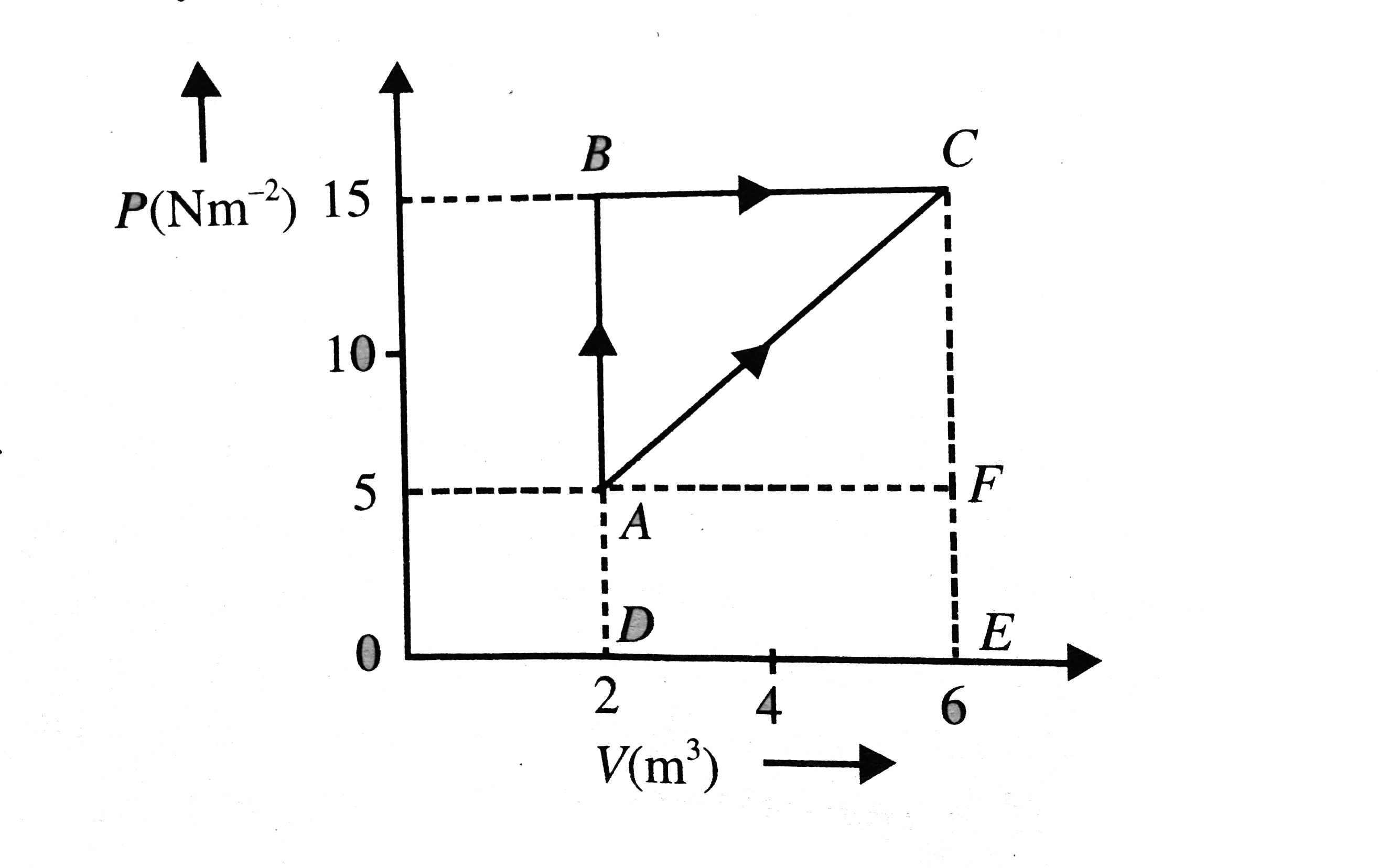
View SolutionThe given figure shows a change of state A to state C by two paths ABC and AC for an ideal gas. Calculate. a. The path along which work done is least. b. The internal energy at C if the internal energy of gas at A is 10J and amount of heat supplied to change its state to C through the path AC is 200J . c. The amount of heat supplied to the gas to go from A to B , if internal energy of gas at state B is 10J .
View SolutionThe internal energy of a gas is given by U = (PV)/2 . At constant volume, state of gas changes from (P_0,V_0) to (3P_0,V_0) then
View SolutionIn the given figure, an ideal gas changes it state from A to state C by two paths ABC and AC . (i) Find the path along which work done is the least. (ii) The internal energy of the gas at A to 10J and the amount of heat supplied to change its state to C through the path AC is 200J . Calculate the internal energy at C . (iii) The internal energy of the gas at state B is 30J . Find the amount of heat supplied to the gas to go from A to B .
View Solution

