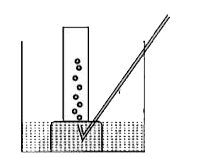
A metal is treated with dilute sulphuric acid. The gas evolved is collected by the method shown in the figure:

(i) Name the gas
(ii) Is the gas soluble or insoluble in water?
(iii) Is the gas lighter or heavier than air?
(iv) How will you test the gas
(v) IF the metal used above in zinc then write the chemical equation for the evolution of gas
(vi) Write one industrial use of the gas evolved.Solution in Kannada
(i) Hydrogen gas
(ii) Is soluble in water
(iii) Gas is lighter than air.
(iv) Test for H2 gas: Bring a burning matchstick near the gas jar. It burns with a pop sound.
(v) Zn(s)+H2SO4(dil)→ZnSO4(aq)+H2(g)↑⏐
(vi) Liquid hydrogen is used as a fuel in rockets.
Similar Questions
किसी धातु की तनु H2SO4 अम्ल से क्रिया कराई जाती है। उत्सर्जित गैस में दिखाई विधि से एकत्र किया जाता है। निम्नांकित के उत्तर दे -
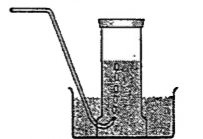
(1) गैस का नाम बताएँ |
(2) गैस को एकत्र करने की विधि का नाम बताएँ।
(3) क्या गैस जल में विलेय है अथवा नहीं ?
(4) क्या गैस वायु से हल्की है अथवा भारी ?
(5) गैस का रासायनिक सूत्र लिखें
View SolutionA metal when treated with dilute sulphuric acid ( H_2SO_4 ) liberates a gas by a method as shown in the figure. Is this gas soluble or insoluble in water?
View SolutionA metal when treated with dilute sulphuric acid ( H_2SO_4 ) liberates a gas by a method as shown in the figure. Is the gas lighter of havier than air?
View SolutionA metal is treated with dilute sulphuric acid. The gas is collected by the method shown in figure ahead. Answer the following:
Name the gas.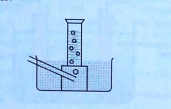
View SolutionA metal is treated with dilute sulphuric acid. The gas is collected by the method shown in figure ahead. Answer the following:
Name the method of collection of the gas.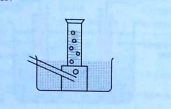
View SolutionA metal is treated with dilute sulphuric acid. The gas evolved is collected by the method shown in the figure:
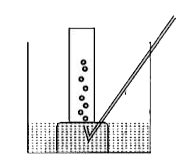
Name the gas evolved:
View SolutionA metal is treated with dilute sulphuric acid. The gas evolved is collected by the method shown in the figure:
The gas evolved is :
View Solution